中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- 金巍, 刘军花, 李袁飞, 成艳芬, 朱伟云. 2017
- Wei Jin, Junhua Liu, Yuanfei Li, Yanfen Cheng, Weiyun Zhu. 2017
- 甲烷菌对厌氧真菌不同碳源代谢的影响
- Effect of methanogens on carbon metabolism of anaerobic fungi
- 微生物学报, 57(7): 1106-1111
- Acta Microbiologica Sinica, 57(7): 1106-1111
-
文章历史
- 收稿日期:2016-11-03
- 修回日期:2017-02-13
- 网络出版日期:2017-03-01
在瘤胃中,厌氧真菌是最先定殖到植物纤维组织上的微生物之一,它能够分泌一系列的水解酶并高效降解植物细胞壁成分。厌氧真菌在瘤胃代谢过程中起着至关重要的作用,但大多数研究都集中于其水解酶系统,鲜有关于其代谢机制及影响因素方面的研究报道。厌氧真菌没有线粒体,利用“氢体”产生ATP[1-2]。在厌氧真菌的代谢途径中,存在2个关键酶丙酮酸甲酸裂解酶(同时存在于细胞质和氢体中)和乙醇脱氢酶E (位于细胞质中),这是它与其他真核微生物显著不同之处[3]。厌氧真菌的代谢产物主要有甲酸、乙酸、乳酸、乙醇、琥珀酸、H2和CO2[4]。其中H2、甲酸和乙酸在氢体中生成,甲酸、乳酸、乙醇和少量琥珀酸在细胞质中生成[3]。厌氧真菌的代谢受底物类型影响。虽然厌氧真菌利用不同碳源的代谢产物基本相同,但各产物的比例会发生变化[5]。碳源对厌氧真菌代谢的影响机制目前还不清楚。
厌氧真菌的代谢产物甲酸、H2和CO2是瘤胃甲烷菌的主要底物[6],这两类微生物之间存在着紧密的种间氢转移关系[7]。厌氧真菌与甲烷短杆菌共培养时,终产物主要有乙酸、甲烷和CO2,还有少量的乙醇和乳酸,氢气和甲酸几乎没有[8]。甲烷菌改变厌氧真菌的代谢途径,与甲烷菌清除厌氧真菌代谢产物H2、解除产物反馈抑制、促进厌氧真菌细胞内碳源流向氢体、增强能量代谢有关。本文通过研究厌氧真菌及厌氧真菌和甲烷菌共培养发酵不同碳水化合物的代谢特点,探讨了影响厌氧真菌碳代谢的因素。
1 材料和方法 1.1 菌种来源本文应用的厌氧真菌和甲烷菌均来源于南京农业大学消化道微生物研究室。共培养F1:Orpinomyces sp. + Methanobrevibacter sp.;共培养N3:Neocallimastix sp. + Methanobrevibacter sp.。
1.2 培养基及培养方法培养基的配制参照朱伟云等[9]描述的方法。发酵采用160 mL血清瓶,100 mL发酵体系,异丁烯盖封口,铝盖固定。每个发酵瓶含培养基95 mL,接种5 mL培养3 d的菌液(每毫升菌液中厌氧真菌ITS1基因拷贝数2×106-5×106),底物添加量0.8%,39 ℃下静置发酵96 h,每处理3个重复。
接种结束后,用气压转换仪(IGER,UK)平衡发酵瓶内气压,使初始气压为零。分别于不同时间点测定产气量和甲烷产量。发酵结束时测定发酵液pH,采集发酵液,-20 ℃保存,备测乙酸、甲酸、乳酸和乙醇。
底物:粉碎玉米芯、木薯粉、大米粉、小麦粉、土豆粉、玉米粉、葡萄糖、麦芽糖、麦芽糊精和可溶性淀粉。淀粉底物的组成成分见表 1。
1.3 总产气量总产气量的测定参照Theodorou等[10]的方法,用气压转换仪定时测定发酵瓶内产气量。将各时间点的产气量相加得到累计产气量。
1.4 发酵终产物分析甲烷的测定参照胡伟莲等[11]的气相色谱法。甲酸检测参照Hopner和Knappe[12]描述的比色法。乳酸检测使用L, D-乳酸试剂盒(南京建成生物工程研究所)。乙酸检测参照秦为琳等[13]描述的气相色谱法。乙醇的测定参照Edgardo等[14]描述的气相色谱方法。
1.5 数据统计数据采用SPSS 20.0软件中的One-way ANOVA (Tukey)进行分析,置信区间为95%。
| Compesition | Cassava | Potato | Corn | Rice | Wheat |
| Dry matter | 87.14 | 88.87 | 86.41 | 86.16 | 86.77 |
| Crude protein | 2.24 | 11.11 | 6.04 | 7.84 | 11.94 |
| Crude ash | 3.31 | 2.88 | 1.57 | 4.88 | 2.44 |
| Crude fiber | 2.50 | 2.30 | 2.10 | 0.23 | 0.11 |
| Ether extract | 0.65 | 0.51 | 0.73 | 0.00 | 0.93 |
| Nitrogen free extract | 78.44 | 72.07 | 75.97 | 73.21 | 71.35 |
| Reducing sugar | 2.75 | 4.77 | 1.37 | 1.37 | 3.10 |
| Starch | 73.02 | 63.62 | 71.35 | 69.99 | 65.51 |
| Amylose | 24.79 | 28.01 | 24.63 | 19.26 | 26.11 |
| Amylopectin | 48.23 | 35.61 | 46.71 | 49.73 | 39.41 |
2 结果和分析 2.1 玉米芯和木薯粉对厌氧真菌以及厌氧真菌和甲烷菌共培养代谢产物的影响
如表 2所示,共培养F1和N3发酵玉米芯和木薯均产生了大量甲烷,甲酸产量低于1.00 mmol/L。有趣的是F1发酵木薯粉[(26.44±0.22) mmol/L]的乳酸产量是发酵玉米芯[(1.31±0.04) mmol/L]的20.18倍,是N3发酵木薯粉[(1.59±0.03) mmol/L]的16.63倍,玉米芯[(0.79±0.08) mmol/L]的33.47倍。厌氧真菌纯培养发酵结果显示,各组甲酸大量积累( > 27.90 mmol/L),乳酸产量均低于1.83 mmol/L,乙醇低于10.40 mmol/L,未检测到甲烷生成。
| Co-cultures | Methane/(mmol/L) | Acetate/(mmol/L) | Formate/(mmol/L) | Lactate/(mmol/L) | Ethanol/(mmol/L) |
| F1+corn core | 12.85±0.80 | 42.88±0.67 | < 1.00 | 1.31±0.04 | 2.48±0.08 |
| F1+cassava | 11.10±0.21 | 37.30±0.89 | < 1.00 | 26.44±0.22 | 14.49±0.06 |
| N3+corn core | 15.69±0.30 | 37.34±0.42 | < 1.00 | 0.79±0.08 | 3.50±0.13 |
| N3+cassava | 18.71±0.22 | 51.41±0.98 | < 1.00 | 1.59±0.03 | 5.11±0.19 |
| F1*+corn core | ND | 16.95±0.52 | 28.21±0.24 | 0.98±0.06 | 10.39±0.09 |
| F1* +cassave | ND | 24.64±0.93 | 27.90±0.09 | 1.83±0.03 | 9.02±0.10 |
| N3* +corn core | ND | 18.94±0.93 | 27.94±0.44 | 0.91±0.00 | 6.26±0.03 |
| N3* +cassave | ND | 28.06±0.42 | 30.12±0.27 | 1.82±0.01 | 7.27±0.04 |
| ND: not detectable; Results are means±SD (n =3); *: pure fungal cultures. | |||||
2.2 增加发酵体系中木薯粉添加量对共培养F1主要代谢终产物的影响
由图 1可见,随着木薯粉添加量的升高,pH逐步下降(P < 0.001)。甲烷、乳酸和乙酸产量均呈先上升后下降的趋势(P < 0.001),暗示代谢途径的转移与底物添加量密切相关。甲烷和乙酸产量最高点出现在木薯粉添加量为1.0%时,乳酸产量最高点出现在木薯粉添加量为2.0%时。木薯粉添加量为2.4%时,乳酸产量下降,暗示过低的pH已经抑制了厌氧真菌的活性,代谢停止。
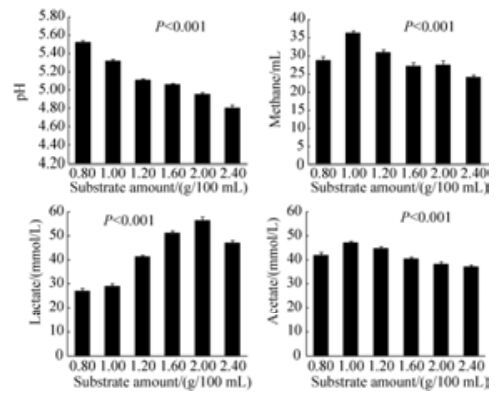
|
| 图 1 F1发酵不同浓度木薯粉主要代谢终产物的产量 Figure 1 The amount of main end-products of F1 incubating on different amount of cassava. |
2.3 不同淀粉底物对共培养F1乳酸产量的影响
如表 3所示,F1发酵5种不同富含淀粉底物的终产物生成量差异较大(P < 0.05)。大米粉产乳酸
| Carbon source | Total gas/mL | Methane/mL | Lactate/(mmol/L) | Acetate/(mmol/L) | Lactate/acetate |
| Rice | 248.3±2.4b | 39.8±0.4c | 36.44±2.35c | 52.44±4.07ab | 0.70±0.07bc |
| Cassava | 217.7±8.0a | 28.6±1.0a | 32.30±4.01bc | 43.11±6.01a | 0.77±0.20c |
| Corn | 246.0±5.3b | 39.5±0.8bc | 27.56±2.35b | 58.67±2.67b | 0.47±0.06ab |
| Wheat | 234.3±3.6b | 37.2±0.6b | 19.26±0.51a | 61.78±2.04b | 0.31±0.01a |
| Potato | 236.9±6.4b | 41.5±1.1c | 15.70±2.57a | 57.78±0.77b | 0.27±0.04a |
| Data were showed as mean±SD (n =3); Significant difference was declared at P < 0.05. | |||||
[(36.44±2.35) mmol/L]和乙酸[(52.44±4.07) mmol/L]较高,土豆粉产甲烷[(41.5±1.1) mmol/L]较高,乳酸[(15.70±2.57) mmol/L]较低。木薯粉总产气量最低[(217.7±8.0) mmol/L],乳酸和乙酸比值较高,暗示木薯粉对F1的代谢影响最大。结合分析表 1和表 3中的数据,如图 2所示,淀粉底物中支链淀粉的比例和乳酸产量呈正相关(R2=0.9554)。

|
| 图 2 共培养F1乳酸产量与淀粉底物中支链淀粉含量相关性分析 Figure 2 The correlation of the lactate yields by F1 and the percentage of amylopectin in the strarch-rich materials. |
2.4 单糖、双糖和多糖对共培养
F1代谢的影响如表 4所示,F1发酵麦芽糊精产生乳酸的量是葡萄糖的12倍,麦芽糖的5.7倍,暗示麦芽糊精能够促进F1的乳酸代谢途径,而葡萄糖和麦芽糖不能。需要注意麦芽糊精虽然增加了乳酸产量,却降低了乙酸的相对产量,暗示共培养F1中厌氧真菌碳代谢途径发生了相对转移。
| Substrate | pH | Methane/mL | Lactate/(mmol/L) | Acetate/(mmol/L) |
| Glucose Maltose Maltodextrin | 5.53±0.01b 5.46±0.01b 5.04±0.01a | 46.18±1.15a 58.15±0.99b 47.36±0.99a | 2.32±0.03a 4.87±0.23a 28.00±0.95b | 53.68±0.65b 66.29±0.73c 45.49±0.74a |
| Data were showed as mean±SD (n =3); Significant difference was declared at P < 0.05. | ||||
3 讨论
深入了解瘤胃厌氧真菌的代谢特点及其影响因素对提高瘤胃代谢效率,提高动物生产性能有重要意义。因此本文研究了厌氧真菌及厌氧真菌和甲烷菌共培养发酵不同碳水化合物的代谢特点,探讨了影响厌氧真菌碳代谢的因素。结果显示碳源类型和甲烷菌改变了厌氧真菌的碳代谢途径。
木薯粉增强了F1乳酸代谢途径,但没有增强N3的乳酸代谢途径,暗示木薯粉对厌氧真菌乳酸代谢途径的增强存在种属特异性。2个共培养中的甲烷菌都是甲烷短杆菌(Methanobrevibacter sp.),F1中的厌氧真菌属于Orpinomyces属,N3中的厌氧真菌属于Neocallimatix属。当厌氧真菌纯培养时,木薯粉未能增强F1和N3的乳酸代谢途径,暗示甲烷菌在增强厌氧真菌乳酸代谢途径中起着重要作用。F1发酵玉米芯、N3发酵玉米芯和木薯的结果与已有研究结果一致,唯有F1发酵木薯与已有研究结果相反。甲烷菌与厌氧真菌共培养一般会增强乙酸代谢途径,减弱乳酸代谢途径[7-8, 15-16]。进一步的研究将验证这种增强作用是否具有普遍性。
Boxma等[3]描绘了厌氧真菌Piromyces sp. E2的代谢图谱。图谱显示,乳酸、乙醇和甲酸在细胞质中生成,氢气、乙酸和甲酸在氢体中生成。作者称这种能量代谢的区域化加强了厌氧真菌代谢调节,并且以果糖为底物验证了这一推断。增加果糖的添加量,Piromyces sp. E2的代谢发生改变,随着果糖添加量的增加,碳代谢逐渐从氢体转向细胞质,表现是乳酸和乙醇产量增加,乙酸和氢气产量相对减少。本文的研究结果显示在木薯粉和甲烷菌共同作用下,F1中厌氧真菌的碳代谢也从氢体转向细胞质,并且增加底物的添加量,碳代谢转移现象增强。这一结果进一步证实了厌氧真菌的碳代谢存在细胞质和氢体之间的动态调节,但这种动态调节的机制目前尚不清楚。
共培养F1发酵5种富含淀粉底物的乳酸产量与底物中支链淀粉的含量呈高度正相关,暗示支链淀粉可能与增强厌氧真菌的乳酸代谢途径有关。支链淀粉高度支链化,有利于快速水解,短时间内可提供厌氧真菌大量可利用糖。但是葡萄糖和麦芽糖均不能增强F1中厌氧真菌的乳酸代谢途径,同时麦芽糊精能够产生与木薯相同的增强效果,暗示短时间内提供大量可利用糖不是增强厌氧真菌乳酸途径的关键因素。下一步工作将利用转录组学技术,期望从转录水平上找到相关信息,揭示厌氧真菌乳酸代谢途径增强的机制。
| [1] | Yarlett N, Orpin CG, Munn EA, Yarlett NC, Greenwood CA. Hydrogenosomes in the rumen fungus Neocallimastix patriciarum. Biochemical Journal, 1986, 236(3): 729-739. DOI:10.1042/bj2360729 |
| [2] | Müller M. The hydrogenosome. Journal of General Microbiology, 1993, 139(12): 2879-2889. DOI:10.1099/00221287-139-12-2879 |
| [3] | Boxma B, Voncken F, Jannink S, Van Alen T, Akhmanova A, van Weelden SWH, Van Hellemond JJ, Ricard G, Huynen M, Tielens AGM, Hackstein JHP. The anaerobic chytridiomycete fungus Piromyces sp. E2 produces ethanol via pyruvate:formate lyase and an alcohol dehydrogenase E. Molecular Microbiology, 2004, 51(5): 1389-1399. DOI:10.1046/j.1365-2958.2003.03912.x |
| [4] | Bauchop T, Mountfort DO. Cellulose fermentation by a rumen anaerobic fungus in both the absence and the presence of rumen methanogens. Applied and Environmental Microbiology, 1981, 42(6): 1103-1110. |
| [5] | 朱伟云. 瘤胃微生物//冯仰廉. 反刍动物营养学. 北京: 科学出版社, 2004. |
| [6] | Hedderich R, Whitman WB. Physiology and biochemistry of the methane-producing archaea//Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E. The Prokaryotes. New York:Springer, 2006. |
| [7] | Jin W, Cheng YF, Mao SY, Zhu WY. Isolation of natural cultures of anaerobic fungi and indigenously associated methanogens from herbivores and their bioconversion of lignocellulosic materials to methane. Bioresource Technology, 2011, 102(17): 7925-7931. DOI:10.1016/j.biortech.2011.06.026 |
| [8] | Li YF, Jin W, Cheng YF, Zhu WY. Effect of the associated methanogen Methanobrevibacter thaueri on the dynamic Profile of end and intermediate metabolites of anaerobic fungus Piromyces sp. F1. Current Microbiology, 2016, 73(3): 434-441. DOI:10.1007/s00284-016-1078-9 |
| [9] |
Zhu WY, Mao SY, Wang QJ, Yao W, Theodorou MK. Study on the screening of anaerobic fungi by in vitro fermentation. Journal of Nanjing Agricultural University, 2001, 24(3): 44-48.
(in Chinese) 朱伟云, 毛胜勇, 王全军, 姚文, TheodorouMK. 厌氧真菌体外发酵筛选技术的研究. 南京农业大学学报, 2001, 24(3): 44-48. |
| [10] | Theodorou MK, Williams BA, Dhanoa MS, McAllan AB, France J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Animal Feed Science and Technology, 1994, 48(3/4): 185-197. |
| [11] |
Hu WL, Wang JK, Lü JM, Guo YQ, Liu JX. Rapid gas chromatogram determination of methane, organic acid in in vitro ruminal fermentation products. Journal of Zhejiang University (Agriculture & Life Sciences), 2006, 32(2): 217-221.
(in Chinese) 胡伟莲, 王佳堃, 吕建敏, 郭嫣秋, 刘建新. 瘤胃体外发酵产物中的甲烷和有机酸含量的快速测定. 浙江大学学报(农业与生命科学版), 2006, 32(2): 217-221. |
| [12] | Hopner T, Knappe J. Formate determination with formate dehydrogenase//Bergmeyer HU. Methods of Enzymatic Analysis. New York:Academic Press, 1974. |
| [13] |
Qin WL. Determination of rumen volatile fatty acids by means of gas chromatography. Journal of Nanjing Agricultural University, 1982, 5(4): 110-116.
(in Chinese) 秦为琳. 应用气相色谱测定瘤胃挥发性脂肪酸方法的研究改进. 南京农业大学学报, 1982, 5(4): 110-116. |
| [14] | Edgardo A, Carolina P, Manuel R, Juanita F, Baeza J. Selection of thermotolerant yeast strains Saccharomyces cerevisiae for bioethanol production. Enzyme and Microbial Technology, 2008, 43(2): 120-123. DOI:10.1016/j.enzmictec.2008.02.007 |
| [15] | Marvin-Sikkema FD, Richardson AJ, Stewart CS, Gottschal JC, Prins RA. Influence of hydrogen-consuming bacteria on cellulose degradation by anaerobic fungi. Applied and Environmental Microbiology, 1990, 56(12): 3793-3797. |
| [16] | Teunissen MJ, Kets EPW, Op den Camp HJM, Huis in't Veld JHJ, Vogels GD. Effect of coculture of anaerobic fungi isolated from ruminants and non-ruminants with methanogenic bacteria on cellulolytic and xylanolytic enzyme activities. Archives of Microbiology, 1992, 157(2): 176-182. |
 2017, Vol. 57
2017, Vol. 57




