中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- 黄丽军, 黄功华, 刘新光. 2017
- Lijun Huang, Gonghua Huang, Xinguang Liu. 2017
- MKP-1在结核分枝杆菌感染中的作用和研究进展
- Role of mitogen-activated protein kinase phosphatase-1 in Mycobacterium tuberculosis infection
- 微生物学报, 57(7): 978-984
- Acta Microbiologica Sinica, 57(7): 978-984
-
文章历史
- 收稿日期:2017-01-06
- 修回日期:2017-02-19
- 网络出版日期:2017-03-03
2. 广东医科大学, 广东省医学分子诊断重点实验室, 广东 东莞 523808;
3. 广东医科大学附属深圳第三医院, 广东 深圳 518020;
4. 上海交通大学医学院, 上海市免疫学研究所, 上海 200025
2. Guangdong Provincial Key Laboratory of Medical Molecular Diagnostics, Guangdong Medical University, Dongguan 523808, Guangdong Province, China;
3. The Affiliated Shenzhen Third Hospital, Guangdong Medical University, Shenzhen 518020, Guangdong Province, China;
4. Shanghai Institute of Immunology, School of Medicine, Shanghai Jiao Tong University, Shanghai 200025, China
结核病(Tuberculosis,TB)是由结核分枝杆菌(Mycobacterium tuberculosis,MTB)引起的慢性传染病,可侵及许多脏器,以肺部结核感染最为常见。据世界卫生组织估计,2015年全世界大约有1040万新发结核病例[1],结核病仍然是威胁人类健康的重大疾病。
在MTB感染过程中,宿主分泌的细胞因子在介导其对MTB的防御反应中发挥重要的作用,如MTB感染的巨噬细胞可分泌炎症因子,介导肉芽肿形成和T细胞免疫反应[2]。在宿主介导MTB感染的细胞内信号转导途径中,有丝分裂原激活的蛋白激酶(mitogen-activated protein kinase,MAPK)是重要的细胞信号通路之一,其包括细胞外信号调节激酶1和2 (Extracellular signal-regulated kinase1/2,ERK1/2),p38 MAPK和c-Jun N-末端激酶(Jun N-terminal kinase,JNK)[3]。当结核分枝杆菌H37Rv感染人源单核细胞后,p38 MAPK和ERK1/2信号通路迅速活化,诱导TNFα的表达,从而在抗结核感染中发挥重要作用[4]。但是,过强的MAPKs活性有时对机体是一种损害,负调控MAPKs活性是维持机体有效免疫反应但不致于产生免疫病理的重要调节方式[5],MAPK磷酸酶(MAPK phosphatases,MKPs)可去除MAPK的磷酸基团而下调MAPK的活性,在调节机体的免疫反应中发挥重要的作用。这些磷酸酶包括酪氨酸磷酸酶,丝氨酸/苏氨酸磷酸酶和双特异性磷酸酶(dual specificity phosphatases,DUSPs),其中DUSPs又被称为MKPs[6]。在所有MKPs中,MKP-1的去磷酸化作用最强[7]。本文就MKP-1在结核分枝杆菌感染中的作用和研究进展做一综述。
1 MKP-1的特性 1.1 MKP-1的发现小鼠MKP-1 cDNA在Balb/c 3T3 cDNA文库中首次被鉴定[8],开始称为3CH134,其氨基酸序列于1992年首次被报道[9]。之后MKP-1的人类同源体(CL100) 也被鉴别出[10]。3CH134与CL100具有相同的酪氨酸磷酸酶催化区域,其催化活性是由MAPK相互作用基序所调节的,因其在体外对ERK1/2的去磷酸化具有高度特异性且是首次报道对MAPK的酪氨酸和苏氨酸残基的去磷酸化,因此命名为MKP-1[11]。体内具有催化活性的MKPs大约有10个,根据其序列同源性、细胞定位和底物特异性大致可分为3类。其中MKP-1被归类为丝裂原和应激诱导型核内MKPs的一种,第二类是胞浆ERK特异性MKPs,第三类是胞浆与胞核JNK/p38特异性MKPs[12]。
1.2 MKP-1的结构与生物学功能MKP-1的蛋白质分子量大小约为40 kDa,其C末端具有3个磷酸酶催化活性位点,包括Asp227、Cys258和Arg264,它们在MKP家族中是高度保守的。而N末端的硫氰酸酶或CDC25样结构域含有关键的激酶相互作用基序(kinase interaction motifs,KIMs),是特异性MAPK底物的结合位点[9]。MKP-1是一种广泛存在于哺乳动物中的细胞核内蛋白,其在心脏、肺和肝脏中表达量最高[11]。尽管MKP-1最初被认为仅对ERK1/2具有特异性,随后的研究表明MKP-1也能使JNK和p38蛋白激酶失活[13]。为了进一步确定MKP-1对底物的特异性,Franklin和Kraft[11]滴定了所建立的U937细胞系中MKP-1表达的水平,发现MKP-1对JNK和p38的特异性可能优于ERKs。同时,在巨噬细胞中敲除MKP-1后,p38和JNK活性增加,但ERK的活性不变[14]。其他研究也发现MKP-1的高表达对p38、JNK和ERK的去磷酸化水平与上述发现具有相似性[14]。在3T3-L1脂肪细胞中,其分化期间,MKP-1表达水平上调,同时ERK1/2水平下调,影响过氧化物酶体增生物激活受体γ (the peroxisome proliferator-activated receptor γ,PPARγ)的活性,其是脂肪形成最主要的调节子[15]。因此,MKP-1对MAPK的调节作用因组织与细胞类型的不同而改变。此外,我们前期的研究发现树突状细胞敲除MKP-1,经LPS刺激后其产生的IL-12p40减少,而IL-6则明显增加;这些细胞因子的变化能被p38抑制剂SB203580纠正,但JNK抑制剂SP600125对这种变化无影响。将MKP-1敲除的树突状细胞与T细胞共培养,T细胞产生IFN-γ减少,但IL-17A与IL-17F产生则明显增加[16]。可见,树突状细胞中MKP-1可通过调节p38 MAPK的活性影响其细胞因子的表达,并进一步调控幼稚T细胞向多种效应性T细胞分化。
2 MKP-1在结核病中的研究进展 2.1 结核分枝杆菌影响巨噬细胞MKP-1的活性及机制研究进展分枝杆菌细胞壁含有大量的糖脂质,包括脂阿拉伯甘露糖(LAM)及其相关前体脂化甘露聚醣(LM),它们可作用于宿主的固有免疫系统[17]。LAM在结核分枝杆菌、牛型分枝杆菌卡介苗(Bacillus Calmette Guerin vaccine,BCG)或堪萨斯分枝杆菌中均存在,其可抑制巨噬细胞的功能,从而促进该病原菌在巨噬细胞内存活[18]。纯化的LM可通过与TLR2结合,并进一步活化其下游的MAPK和NF-kB信号通路,诱导炎症因子的表达,同时也可通过TLR2非依赖方式诱导炎症因子的表达[19]。
最近研究还发现LM与巨噬细胞TLR2结合后除产生TNFα和IL-12[20]外,同时还诱导MKP-1的激活[21]。研究发现堪萨斯分枝杆菌脂化甘露聚醣(Mycobacterium kansasii lipomannan,KanLM)刺激人源单核细胞(THP-1) 可诱导ERK、JNK和p38的活化,但60 min后ERK和p38即失活[22]。这种ERK和p38的活性下调与MKP-1的表达上调相关[21]。当用U0126和SB203580 (它们分别是ERK1/2和p38的抑制剂)处理THP-1后,MKP-1的表达则明显下调,表明ERK和p38在分枝杆菌LM上调MKP-1的表达中具有重要作用,同时ERK和p38的活性又受MKP-1的负反馈调节(图 1)[23]。
之前有研究表明,致病性和非致病性分枝杆菌影响MAPK的磷酸化水平和持续时间可不同[24]。如鸟型分枝杆菌724菌株(一种致病菌)感染鼠源巨噬细胞后,细胞内p38和ERK1/2磷酸化更早但时间更短[25-26],而非致病菌株感染巨噬细胞时,其p38和ERK1/2的磷酸化水平更高,也可持续在24 h以上,同时上调TNFα表达[27]。此外,副结核鸟型分枝杆菌(是一种引起慢性肠道疾病与慢性消耗性疾病的致病性放线菌),通过影响MAPK的活性可诱导比普通型鸟型分枝杆菌更多的IL-10表达以及更少的TNFα表达(图 2)[28]。所有的这些研究表明,结核分枝杆菌可通过调控巨噬细胞MKP-1的活性从而影响MAPK信号通路,可抑制抗结核分枝杆菌因子TNFα的表达和促进炎性抑制因子IL-10的表达,这很可能是结核分枝杆菌得以长期在巨噬细胞生存的重要机制。
2.2 细胞内MKP-1在抗结核感染免疫中的机制
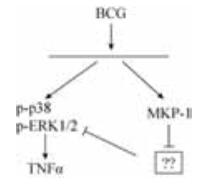
BCG是一种分枝杆菌,常用于研究机体的抗MTB的免疫应答反应机制[29-30]。如BCG刺激人外周血单核细胞(peripheral blood mononuclear cell,PBMC),可活化MAPK和NF-kB信号通路,诱导TNFα的表达[31]。同时BCG刺激PBMC也能活化MKP-1,进一步研究发现BCG诱导的MKP-1的表达依赖于ERK1/2和p38 MAPK信号通路的活化[32],但抑制ERK1/2和p38的活性对MKP-1的表达影响不大,表明还有其他物质参与BCG诱导的MKP-1的表达。通过siRNA下调人原代单核细胞(primary human blood monocytes,PBMO)中MKP-1的基因表达后,发现BCG诱导的MAPK的活性与TNFα的表达均下降,表明在BCG诱导的MAPK的活化和TNFα的表达中,MKP-1是其上游信号分子(图 3)[32]。
此外,BCG诱导的ERK1/2磷酸化可稳定MKP-1蛋白的结构[33]。然而,关于MKP-1在BCG诱导的TNFα表达的调节机制研究中,有报道显示双特异性磷酸酶2 (DUSP2) 与MKP-1一样,可促进BCG诱导的TNFα表达[34-35],其机制是BCG诱导DUSP2的活化,抑制JNK的激活,进一步活化ERK1/2和p38信号通路,上调TNFα的表达(图 4)[34]。
由于MKP-1和DUSP2的结构是相似的,同时它们都含有MAPK相互作用结构域和磷酸酶催化位点[35],因此我们猜测MKP-1也是通过抑制JNK的活性来激活ERK1/2和p38信号通路以及上调TNFα的表达。但在BCG诱导的MKP-1表达中,JNK磷酸化水平并不增加[34],因此关于MKP-1在BCG诱导的TNFα表达的调节机制中,其详细过程需进一步研究(图 3)。有研究发现p38 MAPK信号通路的活化可抑制结核分枝杆菌吞噬体的成熟[36]以及减少核内体与吞噬体间的膜束缚分子的迁移[37]。Souza等也发现抑制p38 MAPK的活性后可增加吞噬体酸化和单核细胞对副结核鸟型分枝杆菌的杀菌能力[38]。此外,Pennini等人发现ERK1/2和p38的信号传导与19 kDa脂蛋白(一种分枝杆菌TLR2激动剂)抑制MHC Ⅱ类反式激活因子(class Ⅱ transactivator,CIITA)的表达有关[39]。因此,p38 MAPK和ERK1/2信号通路对巨噬细胞的抗结核分枝杆菌感染和抗原呈递发挥重要的调节作用,有助于逃避宿主的免疫应答。所有的这些研究表明,MKP-1在抗结核免疫中具有重要作用,但其详细机制有待进一步研究。
3 问题和展望在结核杆菌感染时,分枝杆菌LM如何刺激巨噬细胞MKP-1的表达,从而使其长期在巨噬细胞生存的作用尚不清楚。同时,宿主细胞MKP-1如何在抗结核杆菌感染中发挥重要的免疫调节作用的机制也不明确。由于MKP-1在调节宿主免疫反应中发挥着极其重要的作用,用MKP-1作为靶分子来设计药物是近年来研究的热点[40]。虽然目前研究证明MKP-1参与了抗结核杆菌感染的免疫应答,但结核病的发生是致病菌和宿主免疫细胞之间相互作用的一个复杂过程,由于研究工作主要是在体外实验获得,不同研究组的结果也不一致。因此,MKP-1在抗结核杆菌感染中的作用还需进一步探讨。未来人们可以采用MKP-1条件性敲除小鼠或过表达的永生化细胞,来研究不同类型细胞中MKP-1在抗结核分枝杆菌中的作用及机制,这些研究成果将为结核病的诊断与治疗开辟新的方向。
| [1] | Mario R, Giorgia S. Tuberculosis 2015:burden, challenges and strategy for control and elimination. Infectious Disease Reports, 2016, 8(2): 6570. |
| [2] | Khan N, Vidyarthi A, Javed S, Agrewala JN. Innate immunity holding the flanks until reinforced by adaptive immunity against Mycobacterium tuberculosis infection. Frontiers in Microbiology, 2016, 7: 328. |
| [3] | McClean CM, Tobin DM. Macrophage form, function, and phenotype in mycobacterial infection:lessons from tuberculosis and other diseases. Pathogens and Disease, 2016, 74(7): ftw068. DOI:10.1093/femspd/ftw068 |
| [4] | Zhang Y, Wahl LM. Cytokine-induced monocyte MMP-1 is negatively regulated by GSK-3 through a p38 MAPK-mediated decrease in ERK1/2 MAPK activation. Journal of Leukocyte Biology, 2015, 97(5): 921-927. DOI:10.1189/jlb.3A0413-235R |
| [5] | Arthur JS, Ley SC. Mitogen-activated protein kinases in innate immunity. Nature Reviews Immunology, 2013, 13(9): 679-692. DOI:10.1038/nri3495 |
| [6] | Huang CY, Tan TH. DUSPs, to MAP kinases and beyond. Cell & Bioscience, 2012, 2(1): 24. |
| [7] | Lloberas J, Valverde-Estrella L, Tur J, Vico T, Celada A. Mitogen-activated protein kinases and mitogen kinase phosphatase 1:A critical interplay in macrophage biology. Frontiers in Molecular Biosciences, 2016, 3: 28. |
| [8] | Lau LF, Nathans D. Identification of a set of genes expressed during the G0/G1 transition of cultured mouse cells. The EMBO Journal, 1985, 4(12): 3145-3151. |
| [9] | Charles CH, Abler AS, Lau LF. cDNA sequence of a growth factor-inducible immediate early gene and characterization of its encoded protein. Oncogene, 1992, 7(1): 187-190. |
| [10] | Keyse SM, Emslie EA. Oxidative stress and heat shock induce a human gene encoding a proteintyrosine phosphatase. Nature, 1992, 359(6396): 644-647. DOI:10.1038/359644a0 |
| [11] | Zheng CF, Guan KL. Dephosphorylation and inactivation of the mitogen-activated protein kinase by a mitogen-induced Thr/Tyr protein phosphatase. Journal of Biological Chemistry, 1993, 268(22): 16116-16119. |
| [12] | Caunt CJ, Keyse SM. Dual-specificity MAP kinase phosphatases (MKPs):shaping the outcome of MAP kinase signalling. The FEBS Journal, 2013, 280(2): 489-504. DOI:10.1111/febs.2013.280.issue-2 |
| [13] | Rastogi R, Jiang Z, Ahmad N, Rosati R, Liu Y, Beuret L, Monks R, Charron J, Birnbaum MJ, Samavati L. Rapamycin induces mitogen-activated protein (MAP) kinase phosphatase-1(MKP-1) expression through activation of protein kinase B and mitogen-activated protein kinase kinase pathways. Journal of Biological Chemistry, 2013, 288(47): 33966-33977. DOI:10.1074/jbc.M113.492702 |
| [14] | Kjellerup RB, Johansen C, Kragballe K, Iversen L. The expression of dual-specificity phosphatase 1 mRNA is downregulated in lesional psoriatic skin. British Journal of Dermatology, 2013, 168(2): 339-345. DOI:10.1111/bjd.12020 |
| [15] | So YG, Ji YA, Chang HJ, Bo KM, Tae YH. Shikonin suppresses ERK 1/2 phosphorylation during the early stages of adipocyte differentiation in 3T3-L1 cells. BMC Complementary and Alternative Medicine, 2013, 13: 207. DOI:10.1186/1472-6882-13-207 |
| [16] | Huang G, Wang Y, Shi LZ, Chi H. Signaling by the phosphatase MKP-1 in dendritic cells imprints distinct effector and regulatory T cell fates. Immunity, 2011, 35(1): 45-58. DOI:10.1016/j.immuni.2011.05.014 |
| [17] | Ishikawa E, Mori D, Yamasaki S. Recognition of mycobacterial lipids by immune receptors. Trends in Immunology, 2017, 38(1): 66-76. DOI:10.1016/j.it.2016.10.009 |
| [18] | Venkatasubramanian S, Tripathi D, Tucker T, Paidipally P, Cheekatla S, Welch E, Raghunath A, Jeffers A, Tvinnereim AR, Schechter ME, Andrade BB, Mackman N, Idell S, Vankayalapati R. Tissue factor expression by myeloid cells contributes to protective immune response against Mycobacterium tuberculosis infection. European Journal of Immunology, 2016, 46(2): 464-479. DOI:10.1002/eji.201545817 |
| [19] | Yihao D, Hongyun H, Maodan T. Latency-associated protein Rv2660c of Mycobacterium tuberculosis augments expression of proinflammatory cytokines in human macrophages by interacting with TLR2. Infectious Diseases (London), 2015, 47(3): 168-177. DOI:10.3109/00365548.2014.982167 |
| [20] | Su H, Zhu S, Zhu L, Huang W, Wang H, Zhang Z, Xu Y. Recombinant lipoprotein Rv1016c derived from Mycobacterium tuberculosis is a TLR-2 ligand that induces macrophages apoptosis and inhibits MHC Ⅱ antigen processing. Frontiers in Cellular and Infection Microbiology, 2016, 6: 147. |
| [21] | Elass E, Coddeville B, Kremer L, Mortuaire M, Mazurier J, Guérardel Y. Mycobacterial lipomannan induces MAP kinase phosphatase-1 expression in macrophages. FEBS Letters, 2008, 582(3): 445-450. DOI:10.1016/j.febslet.2008.01.007 |
| [22] | Wang J, McIntosh F, Radomski N, Dewar K, Simeone R, Enninga J, Brosch R, Rocha EP, Veyrier FJ, Behr MA. Insights on the emergence of Mycobacterium tuberculosis from the analysis of Mycobacterium kansasii. Genome Biology and Evolution, 2015, 7(3): 856-870. DOI:10.1093/gbe/evv035 |
| [23] | Isabelle V, Martine G, Jérôme N. Manipulation of the endocytic pathway and phagocyte functions by Mycobacterium tuberculosis lipoarabinomannan. Frontiers in Cellular and Infection Microbiology, 2014, 4: 187. |
| [24] | Souza CD. Blocking the mitogen activated protein kinase-p38 pathway is associated with increase expression of nitric oxide synthase and higher production of nitric oxide by bovine macrophages infected with Mycobacterium avium subsp. paratuberculosis. Veterinary Immunology and Immunopathology, 2015, 164(1/2): 1-9. |
| [25] | Haug M, Awuh JA, Steigedal M, Frengen Kojen J, Marstad A, Nordrum IS, Halaas Ø, Flo TH. Dynamics of immune effector mechanisms during infection with Mycobacterium avium in C57BL/6 mice. Immunology, 2013, 140(2): 232-243. DOI:10.1111/imm.2013.140.issue-2 |
| [26] | Awuh JA, Haug M, Mildenberger J, Marstad A, Do CP, Louet C, Stenvik J, Steigedal M, Damås JK, Halaas Ø, Flo TH. Keap1 regulates inflammatory signaling in Mycobacterium avium-infected human macrophages. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(31). |
| [27] | Chen T, Zhao Q, Li W, Xie J. Mycobacterium tuberculosis PE_PGRS17 promotes the death of host cell and cytokines secretion via Erk kinase accompanying with enhanced survival of recombinant Mycobacterium smegmatis. Journal of Interferon & Cytokine Research, 2013, 33(8): 452-458. |
| [28] | Abendaño N, Juste RA, Alonso-Hearn M. Anti-inflammatory and antiapoptotic responses to infection:a common denominator of human and bovine macrophages infected with Mycobacterium avium subsp. paratuberculosis. BioMed Research International, 2013, 2013: 908348. |
| [29] | Tzvetelina S. Quality control and safety assessment of BCG vaccines in the post-genomic era. Biotechnology & Biotechnological Equipment, 2014, 28(3): 387-391. |
| [30] | Alice L, Sunil J. Improving the immunogenicity of the Mycobacterium bovis BCG vaccine by non-genetic bacterial surface decoration using the Avidin-Biotin system. PLoS One, 2015, 10(12): e0145833. DOI:10.1371/journal.pone.0145833 |
| [31] | Galbadage T, Shepherd TF, Cirillo SL, Gumienny TL, Cirillo JD. The Caenorhabditis elegans p38 MAPK gene plays a key role in protection from Mycobacteria. Microbiologyopen, 2016, 5(3): 436-452. DOI:10.1002/mbo3.341 |
| [32] | Cheung BK, Yim HC, Lee NC, Lau AS. A novel anti-mycobacterial function of mitogen-activated protein kinase phosphatase-1. BMC Immunology, 2009, 10: 64. DOI:10.1186/1471-2172-10-64 |
| [33] | Mattingly HH, Chen JJ, Arur S, Shvartsman SY. A transport model for estimating the time course of ERK activation in the C. elegans. Germline. Biophysical Journal, 2015, 109(11): 2436-2446. DOI:10.1016/j.bpj.2015.10.021 |
| [34] | Crowell S, Wancket LM, Shakibi Y, Xu P, Xue J, Samavati L, Nelin LD, Liu Y. Post-translational regulation of mitogen-activated protein kinase phosphatase (MKP)-1 and MKP-2 in macrophages following lipopolysaccharide stimulation:the role of the C termini of the phosphatases in determining their stability. Journal of Biological Chemistry, 2014, 289(42): 28753-28764. DOI:10.1074/jbc.M114.591925 |
| [35] | Cornell TT, Fleszar A, McHugh W, Blatt NB, Le Vine AM, Shanley TP. Mitogen-activated protein kinase phosphatase 2, MKP-2, regulates early inflammation in acute lung injury. American Journal of Physiology-Lung Cellular and Molecular Physiology, 2012, 303(3): L251-L258. DOI:10.1152/ajplung.00063.2012 |
| [36] | Seto S, Tsujimura K, Koide Y. Coronin-1a inhibits autophagosome formation around Mycobacterium tuberculosis-containing phagosomes and assists mycobacterial survival in macrophages. Cellular Microbiology, 2012, 14(5): 710-727. DOI:10.1111/cmi.2012.14.issue-5 |
| [37] | Fratti RA, Chua J, Deretic V. Induction of p38 mitogen-activated protein kinase reduces early endosome autoantigen 1(EEA1) recruitment to phagosomal membranes. Journal of Biological Chemistry, 2003, 278(47): 46961-46967. DOI:10.1074/jbc.M305225200 |
| [38] | Souza CD, Evanson OA, Weiss DJ. Mitogen activated protein kinase p38 pathway is an important component of the anti-inflammatory response in Mycobacterium avium subsp. paratuberculosis-infected bovine monocytes. Microbial Pathogenesis, 2006, 41(2/3): 59-66. |
| [39] | Kumar P, Agarwal R, Siddiqui I, Vora H, Das G, Sharma P. ESAT6 differentially inhibits IFN-γ-inducible class Ⅱ transactivator isoforms in both a TLR2-dependent and -independent manner. Immunology and Cell Biology, 2012, 90(4): 411-420. DOI:10.1038/icb.2011.54 |
| [40] | Candas D, Lu CL, Fan M, Chuang FY, Sweeney C, Borowsky AD, Li JJ. Mitochondrial MKP1 is a target for therapy-resistant HER2-positive breast cancer cells. Cancer Research, 2014, 74(24): 7498-7509. DOI:10.1158/0008-5472.CAN-14-0844 |
 2017, Vol. 57
2017, Vol. 57