中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- 杨旭, 邢志林, 张丽杰. 2017
- Xu Yang, Zhilin Xing, Lijie Zhang. 2017
- 填埋场氯代烃生物降解过程的机制转化与调控研究及展望
- Advances in transformation and regulation biodegradation of chorinated hydrocarbons in landfill
- 微生物学报, 2017, 57(4): 468-479
-
文章历史
- 收稿日期:2016-09-19
- 修回日期:2016-11-04
- 网络出版日期:2016-11-29
2. 重庆大学城市建设与环境工程学院, 重庆 400045;
3. 重庆理工大学药学与生物工程学院, 重庆 400050
2. College of Urban Construction and Environmental Engineering, Chongqing University, Chongqing 400045, China;
3. School of Pharmacy and Bioengineering, Chongqing University of Technology, Chongqing 400050, China
垃圾填埋场作为国内外的主要固体废弃物处理处置方式,具有安全性高、处理费用低和处理量大等特点[1]。填埋场运行过程中产生的填埋气,是填埋垃圾稳定化过程中的主要副产物[2]。填埋气中除了甲烷 (CH4) 和二氧化碳 (CO2),还包括一些多碳烷烃、环烷烃、芳烃和卤代化合物等挥发性有机物[3-4],其中的氯代烃类污染物种类多、成分复杂、毒性强,如未经处理排放到大气中对周围环境及人类生命造成极大危害[4-6]。因此有效去除该类污染物对地球生态环境的保护有重要意义。
填埋场覆盖土在填埋气长期驯化过程中衍生了多种功能微生物,它们能够有效去除填埋气中甲烷、氯代烃等有毒有害气体[7-9]。前期研究主要报道了覆盖土中甲烷氧化菌在单因素控制条件下对氯代烃的降解作用[10],发现在以甲烷为底物的有氧共代谢条件下,甲烷氧化菌能够降解多种氯代烃[11]。甲烷单加氧酶可将氯代烯烃催化成环氧化合物,而后其自发分解为氯离子和二氧化碳;氯代烷烃在甲烷单加氧酶作用下一般先形成氯代羟基化合物,然后自发脱氯形成氯盐和二氧化碳[12-15]。填埋场场地监测和实验室覆盖土氯代烃降解研究发现,在甲烷为共代谢底物条件下,所有低取代卤代烃都能被降解;有氧甲烷共代谢条件下全卤代烃不能降解,但可进行厌氧脱氯,而后实现共代谢降解[2, 8, 16-17]。
课题组前期开展了填埋场覆盖层中氯代烃共代谢降解的研究,并对不同深度覆盖层中的微生物群落结构和生物多样性进行分析,发现除甲烷外还有多种底物和多种属微生物参与了氯代烃生物降解[18]。受氧气扩散及覆盖层中生物氧化的影响,覆盖层可根据氧气含量分为厌氧区 ( > 40 cm)、兼性厌氧区 (20-40 cm) 和有氧区 (0-20 cm)[19]。现有研究主要针对单一菌种 (如甲烷氧化菌)、单一环境条件 (好氧或厌氧) 的氯代烃生物降解[11, 20-21],但实际上氯代烃在覆盖土中的生物降解存在多种途径且不同的降解机制会随着氧气含量的变化而发生转变。对类似复杂环境体系下氯代烃污染物降解机制及不同工况条件下主导机制转化的系统性归纳目前还未见报道。
据此,本文对多地区填埋场中填埋气的氯代烃污染特性开展广泛调研,系统分析填埋气中氯代烃的种类、浓度变化规律;对氯代烃含量及其降解研究进行总结,确定不同覆盖层深度、不同工况条件下微生物对氯代烃的降解方式及降解能力;结合填埋场覆盖层中氧气分布特性和微生物多样性研究,构建氯代烃在覆盖层底部扩散至大气界面全过程的生物降解机制模型。最后对复合污染场地中氯代烃类污染物的生物降解研究进行展望,以期为复合污染场地难降解污染物的原位生物修复提供理论指导。
1 填埋场中氯代烃的组成填埋气中挥发性氯代烃约占非甲烷类挥发性有机物的2%[9],对现有关于填埋气成分研究的多篇文献中共有的氯代烃的组成和含量进行了统计学分析,其主要种类及浓度变化箱图如图 1所示。填埋气中氯代烃主要以短链 (C < 2) 氯代脂肪烃和氯苯 (一氯,二氯和三氯) 为主。填埋场表层氯代烃平均浓度较高的物质主要是二氯甲烷 (DCM)、三氯甲烷 (TCM)、三氯乙烯 (TCE) 和四氯乙烯 (PCE)[9, 16, 22-23]。其浓度范围分别为0.2-32.4、0.5-32.4、0.60-46.46和2.000-105.045 μg/m3,平均浓度分别为12.99、11.88、10.65和15.46 μg/m3。氯代芳香族化合物普遍含量较少,并较稳定,变化范围为0.50-2.37 μg/m3。由于填埋场的地域差异、气候变化及内部垃圾发酵程度不同,这些物质的浓度变化较大。此外,由于不同地区发展程度及垃圾分类等处理方法的差异性,使得填埋的垃圾组成也有很大差异,导致分解后产生的氯代烃种类各不相同。甚至有研究者在填埋气中检测到的氯代烃超过30种[24]。实际场地分析发现不同深度的氯代烃浓度有很大差别,相关研究发现填埋气中挥发性氯代烃在覆盖层以下50 cm左右时浓度最高[23],氯代烃浓度与深度成正相关,这说明氯代烃在覆盖土中不断发生降解。

|
| 图 1. 填埋气中常见挥发性氯代烃化合物含量变化箱图 Figure 1. The content of common volatile chlorinated hydrocarbon compounds in landfill gas. |
2 覆盖土对氯代烃的生物降解
填埋场覆盖层对甲烷氧化和生物降解氯代烃类物质有巨大潜力[9]。研究者从多方面研究了覆盖土对氯代烃的降解[6, 8, 24],包括血清瓶小试实验、模拟覆盖层氯代烃生物降解和实际场地中氯代烃浓度的监测。不同工况条件覆盖土对氯代烃的降解如表 1所示。氯代烃降解速率受温度、初始浓度、覆盖材料和氧气浓度等因素影响。当温度为22 ℃、甲烷浓度为15%(V/V)、氧气浓度为35% (V/V) 时,通过批次实验发现覆盖土对氯乙烯 (VC) 的降解速率高达8.6 μg/(gsoil·h)[25]。Scheutz等长期研究发现,覆盖土对填埋气中所有的氯代烃均有降解效果,在模拟覆盖土的土柱实验中DCM的去除率高达70%-80%[7, 26]。覆盖土对TCE、DCM等13种氯代烃有明显的生物降解,但当TCE和二氯乙烯 (DCE) 等氯代烃浓度大于20 μg/L时,覆盖土对其几乎没有降解效果。
| Chlorinated hydrocarbons | Concentration/(μg/L) | Biodegradation rates | Experiment condition | References |
| Tetrachloroethylene (PCE) |
7 | 0.025 | 15% CH4 and 35% O2 | [29] |
| 20-2000 | no degradation | 15% CH4 and 35% O2 | [30] | |
| 5 | no degradation | 15% CH4 and 35% O2 | [8] | |
| 30 | no degradation | 15% CH4 and 30% O2 | [16] | |
| 20 | no degradation | 15% CH4 and 30% O2 | [25] | |
| Trichloroethylene (TCE) |
30 | 0.094 | 15% CH4 and 35% O2 | [29] |
| 40 | 0.057±0.153 | 15% CH4 and 35% O2 | [8, 26] | |
| 100 | (4.1±10-2) g/(m2·d) | Column experiments | [26] | |
| 20-2000 | 0.060 | 15% CH4 and 35% O2 | [30] | |
| 30 | 0.057±0.002 | 15% CH4 and 35% O2 | [16] | |
| 50 | 0.017 | 15% CH4 and 30% O2 | [25] | |
| 17.6 | 94.230 mg/(m2·d) | mixed compost (30%) | [31] | |
| 88.110 mg/(m2·d) | mixed compost (50%) | |||
| 83.880 mg/(m2·d) | mixed compost (75%) | |||
| 1, 1-dichloroethylene (1, 1-DCE) |
80 | 0.114 | 15% CH4 and 35% O2 | [29] |
| 80 | 0.050±0.116 | 15% CH4and 35% O2 | [8] | |
| 500 | 0.008 | 15% CH4and 30% O2 | [25] | |
| c-1, 2-dichloroethylene (c-1, 2-DCE) |
400 | 1.381 | 15% CH4 and 35% O2 | [29] |
| 20-2000 | 1.200 | 15% CH4and 35% O2 | [30] | |
| 170 | 0.318±0.078 | 15% CH4and 35% O2 | [8] | |
| 800 | 4.134±0.048 | 15% CH4and 30% O2 | [16] | |
| 800 | 0.225 | 15% CH4and 30% O2 | [25] | |
| t-1, 2-dichloroethylene (t-1, 2-DCE) |
700 | 3.244 | 15% CH4and 35% O2 | [29] |
| 20-2000 | 2.900 | 15% CH4and 35% O2 | [30] | |
| 300 | 1.119±0.015 | 15% CH4and 35% O2 | [8] | |
| 1200 | 1.841±0.013 | 15% CH4and 30% O2 | [16] | |
| 800 | 0.263 | 15% CH4and 30% O2 | [25] | |
| Vinyl chloride (VC) |
120 | 0.509 | 15% CH4and 35% O2 | [29] |
| 1000 | 1.456±0.012 | 15% CH4and 35% O2 | [8, 26] | |
| 310 | 1.8±0.1 | Column experiments | [26] | |
| 20-2000 | 8.600 | 15% CH4and 35% O2 | [30] | |
| 600 | 8.564±0.385 | 15% CH4and 30% O2 | [16] | |
| 100 | 0.175 | 15% CH4and 30% O2 | [25] | |
| Trichloromethane (TCM) |
30 | 0.028±0.005 | 15% CH4and 35% O2 | [8, 26] |
| 50 | (2.30±0.01) g/(m2·d) | Column experiments | [26] | |
| 160 | 0.136±0.004 | 15% CH4and 30% O2 | [16] | |
| 400 | 0.013 | 15% CH4 and 30% O2 | [25] | |
| Dichloromethane (DCM) |
200 | 0.686±0.052 | 15% CH4 and 35% O2 | [8, 26] |
| 1270 | 7.3±0.1 | Column experiments | [26] | |
| 700 | 0.885±0.004 | 15% CH4 and 30% O2 | [16] | |
| 70 | 0.467 | 15% CH4 and 30% O2 | [25] | |
| 54.2 | 427.815 mg/(m2·d) | mixed compost (30%) | [31] | |
| 311.670 mg/(m2·d) | mixed compost (50%) | |||
| 294.300 mg/(m2·d) | mixed compost (75%) | |||
| Tetrachloromethane (TeCM) | 20 | no degradation | 15% CH4and 30% O2 | [16] |
| 1, 1, 1-trichloroethane (1, 1, 1-TCA) | 45 | no degradation | 15% CH4and 35% O2 | [8] |
| 1, 1, 2-trichloroethane (1, 1, 2-TCA) | 40 | 0.136±0.011 | 15% CH4and 35% O2 | [8] |
| 1, 1-dichloroethane (1, 1-DCA) |
260 | 0.169±0.023 | 15% CH4and 35% O2 | [8] |
| 800 | 1.742±0.017 | 15% CH4and 30% O2 | [16] | |
| 1000 | 0.154 | 15% CH4and 30% O2 | [25] | |
| 1, 2-dichloroethane (1, 2-DCA) |
270 | 1.716±0.085 | 15% CH4and 35% O2 | [8] |
| 280 | 2.809±0.089 | 15% CH4and 30% O2 | [16] | |
| 200 | 0.217 | 15% CH4and 30% O2 | [25] | |
| All the experiment were operated in the 22 ℃, the unit of biodegradation rates unsigned were μg/(gsoil/h). | ||||
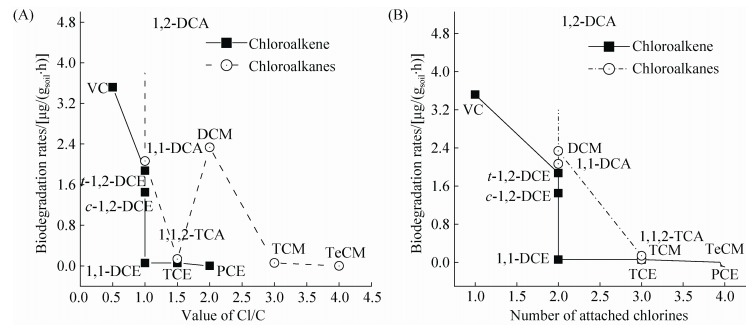
氯代烃结构尤其是氯原子数量对其生物降解机制有很大影响[27],氯代烃中氯原子数及Cl/C对其在覆盖土中降解速率影响如图 2所示。填埋场覆盖土在氧气和甲烷存在条件下,对低氯代化合物降解速率较高。一般而言,覆盖土对氯代烃降解速率随着氯原子取代的增多而降低,氯代烷烃大于氯代烯烃的降解速率。氯原子取代位置不同降解速率也有微小差异,例如反-1, 2-二氯乙烯 (t-1, 2-DCE) 的降解速率高于顺-1, 2-二氯乙烯 (c-1, 2-DCE)。不同氯代烃的降解速率随着Cl/C的比值增大而降低,当Cl/C的比值大于3时污染物的降解速率几乎为0。Hazen[28]也在厌氧和好氧条件下对甲烷氧化菌降解氯代烃速率与取代氯原子数目的关系进行了相关研究,发现氯代烃的氧化速率随氯原子取代数目的增多而降低,还原速率随氯原子取代数目的增加而增加。
|
| 图 2. 氯代烃中氯原子数及Cl/C对其在覆盖土中降解速率影响 Figure 2. The relationship of chlorinated hydrocarbons in landfill gas biodegradation rates between the value of Cl/C (A) and the number of attached chlorine atoms (B). |
3 填埋场覆盖层中氯代烃的生物降解途径 3.1 氯代烃的共代谢生物降解
在自然环境中,氯代烃的主要降解方式之一是共代谢降解[32]。目前发现产生共代谢酶的微生物主要有甲烷氧化菌、假单胞菌属、分支杆菌属和黄色杆菌属。许多烃类 (烷烃类、烯烃和一些芳香烃)、醇类 (甲醇、乙醇)、酚类 (苯酚)、糖类 (葡萄糖)、盐类 (甲酸盐、乙酸盐) 甚至氨都可作为微生物底物共代谢降解氯代烃[28, 33-36]。共代谢降解氯代烃酶主要有烷烃单加氧酶、烯链单加氧酶、甲苯单加氧酶/双加氧酶、氨氧化酶和苯酚羟化酶等[33-47]。
覆盖土中甲烷氧化菌能够以填埋气中甲烷为底物共代谢降解氯代烃已被广泛报道。填埋气中除甲烷外,还有可作为微生物生长底物的丁烷、丙烯等长链脂肪烃和苯、甲苯等芳香烃化合物[2, 4]。已有研究证明覆盖土能有效降解甲苯[48-50],说明覆盖土中存在以非甲烷为底物生长的微生物。高艳辉等[18]研究了铜离子在覆盖土微生物降解TCE中的影响,分析了降解过程中的关键酶的定量表达,发现不仅有甲烷单加氧酶随铜离子变化,还有苯酚羟化酶基因的表达,表达量 (LmpH/16S) 为4.80×10-8-2.22×10-7;同时,生物多样性分析显示降解过程的活性菌除甲烷氧化菌外还有假单胞菌等可共代谢降解氯代烃的微生物。因此,覆盖层中的氯代烃共代谢降解是多种底物,多种微生物和多种酶共同参与的结果。
3.2 氯代烃的直接降解作用有氧条件下,一些微生物可以直接利用某些氯代烃作为能源和碳源。Kitayama首次报道了好氧条件下Pseudomonas aeruginosa JI104能将三氯乙烯作为唯一碳源[51]。Olaniran等[52]在非洲污染场地分离的土著菌能够在有氧条件下对顺反-二氯乙烯 (c-1, 2-DCE,t-1, 2-DCE) 进行还原脱氯。Schmidt等的研究发现从污染地下水中分离的菌在不添加任何生长基质的条件下也能降解TCE[52]。另外,Olaniran报道了分离的7种细菌可以c-1, 2-DCE和t-1, 2-DCE为能源物质[52]。这些分离得到的细菌,有2种属于Acinetobacter species,另外2种为Bacillus species,分别为Bacillus subtilis和Bacillus cereus。
填埋气向上扩散过程中,由于厌氧条件下还原脱氯作用,低氯代烃的种类和含量逐渐增多,在兼性厌氧区和有氧区可以被直接氧化降解。Tiehm等的研究发现氯乙烯 (VC)、t-1, 2-DCE、1, 2-二氯乙烷 (1, 2-DCA) 和DCM等氯代烃可作为微生物碳源[53],相关微生物主要属于Pseudomonas和Bacillus,而覆盖层原始土样中也发现含有丰富的相关菌属[54],孔娇艳等[1]研究发现随着对TCE的降解覆盖土中相关菌属的相对丰度也发生变化。因此直接降解也是填埋气中氯代烃污染物的去除方式之一。
3.3 厌氧条件下氯代烃的还原脱氯氯代烃的生物降解方式与氯代烃的结构及其取代程度有关。有氧条件下,其降解速率随Cl/C值增加而减小,甲烷氧化菌不能降解四氯乙烯 (PCE) 等全氯代化合物[15];厌氧条件下,氯代烃降解更倾向于发生脱氯反应,降解速率随取代程度的增大而增大。目前研究发现有多种微生物能够通过还原脱氯过程将PCE、氯苯等氯代有机物脱氯形成低氯代中间产物或矿化生成CO2和CH4[55]。不同结构的氯代烃脱氯降解过程不同。一般而言,氯代脂肪烃主要通过水解作用、亲核反应和二卤消去作用等机制脱去一个氯原子或多个氯原子;氯代芳香烃则是只能在缺氧条件下由氢取代氯,逐一脱氯的过程。脱卤素酶在厌氧还原脱氯过程中起到了重要作用。
一般填埋场覆盖层覆盖土厚度在70 cm以上,由于氧气扩散不畅和甲烷氧化作用,一般在深度大于40 cm处均为厌氧区。因此在该区域,氯代烃的转化基本以厌氧降解为主。血清瓶实验发现在厌氧条件下,全氯代烃能够被覆盖土降解发生脱氯反应,而有氧时无降解[16]。但关于哪些菌起降解作用还并未有深入研究。
4 覆盖土中与氯代烃降解相关微生物 4.1 甲烷氧化菌填埋场覆盖土中微生物种类十分复杂,何芝等在覆盖土土样中纲分类水平上共检测到80多种微生物,属分类水平上共检测到460多种微生物[54]。在系统发育树上大多属于γ-变形菌门和α-变形菌门的甲烷氧化菌是填埋场覆盖土中最普遍的微生物[56]。不同填埋场中甲烷氧化菌的主要菌属如表 2所示。Ⅰ型甲烷氧化菌主要包括甲基杆菌属、甲基微菌属、甲基单胞菌属、甲基暖菌属、甲基球形菌属、甲基热菌属、甲基八叠球菌属、甲基球菌属和甲基盐菌属等。Ⅱ型甲烷氧化菌主要包括甲基胞囊菌属,甲基弯曲菌属[57-64]。这些甲烷氧化菌将甲烷转化成二氧化碳的第一步都需要合成的甲烷单加氧酶 (MMO) 是共代谢降解填埋气中典型氯代烃的重要催化剂。因此甲烷氧化菌是去除填埋气中甲烷和氯代烃的最主要微生物之一。
| Styles | Methanotrophs | References |
| TypeⅠ | Methylomicrobium | [57-59] |
| Methylococcus | [57-58, 64] | |
| Methylobacter | [58-64] | |
| Methylosarcina | [58-60] | |
| Methylocaldum | [59-60, 63] | |
| Methylomonas | [58-59, 61] | |
| Methylosphaera | [58] | |
| Methylococcales_Unclassified | [58] | |
| TypeⅡ | Methylosinus | [57-58, 60, 62] |
| Methylocystis | [57-59, 61-64] | |
| Methylocella | [58, 61] | |
| Methylocapsa | [58, 61] | |
| Methylocystaceae_Unclassified | [58] |
4.2 其他微生物
现代分子生物学技术分析表明覆盖土含有多种功能微生物,除甲烷氧化菌外,还含有氨氧化菌、硝化细菌、纤维素降解菌、产酸菌、产乙酸菌、产甲烷菌、反硝化细菌、硫氧化菌和硫酸盐还原菌等[65]。赵天涛等通过对覆盖土中的微生物长期研究富集分离出了可降解氯代烯烃的贪铜菌[66]和甲基杆菌[67],它们能够利用三氯乙烯、二氯乙烯和氯乙烯等难降解毒性有机物为唯一碳源和能源生长,能够在贫养环境中保持较高的活性。基于覆盖土微生物,制备了可降解氯代烃复合菌剂[68]。此外,甲烷氧化菌的活性也受其他微生物的影响,相互之间存在多种相互作用关系。Stock等研究并预测了甲烷氧化菌与其他24种异养微生物间的关系,发现在甲基单胞菌属和贪铜菌混合培养时甲烷氧化菌的密度分别是分开培养的3倍和4倍,而铜绿假单胞菌和甲基杆菌混合培养时能够抑制甲烷氧化菌的生长[69]。因此,覆盖土中的氯代烃生物降解并非单一菌种独立完成的,而是多种微生物的共同作用。
5 氯代烃在覆盖土中的降解机制自然界中氯代烃的生物降解过程取决于多种因素,包括共代谢底物类型、微生物种类、氯代烃结构和氧气含量等[31]。生活垃圾填埋场覆盖层作为复杂的次生环境,具有多样的环境体系。首先,从底物多样性而言,填埋气中除甲烷外,还含有芳香烃、多碳烷烃、酮、有机酸、醛、脂类和萜类等[70],这些物质大多数可作为微生物的能源和碳源;其次,覆盖层土壤在填埋气长期驯化过程中衍生了多种微生物,这些菌为氯代烃生物降解提供了丰富的微生物资源;第三,填埋气中检测到的氯代烃种类多,涵盖了氯代烷烃、氯代烯烃和氯代芳烃,结构性差异导致了降解速率和降解方式的不同;最后,覆盖层中甲烷的生物氧化过程和氧气的扩散限制使得覆盖层在不同深度出现了厌氧区、兼性厌氧区和好氧区,这导致了降解方式的差异性。
根据以上分析,填埋气中氯代烃在覆盖土中的生物降解模型如图 3所示。在氯代烃污染物向上扩散的过程中,首先经过覆盖土中的厌氧区,由于氯原子具有强的电负性,多氯代有机物碳原子电子云较低使其在酶的作用下容易与还原剂发生反应[71],可以推测在覆盖土厌氧区的还原脱氯作用是多氯代有机物进行生物降解的第一步。随着挥发性有机物在覆盖层中的扩散,厌氧降解产物和未发生降解的氯代烃进入覆盖层中的兼性厌氧区和有氧区。在该区域低氯取代的化合物在多种微生物作用下发生共代谢降解或直接作为微生物生长基质,氯代脂肪烃在加氧酶的作用下一般先转化为不稳定的环氧化物,破坏有机物结构,然后进一步降解为短链酸等中间产物,从而实现降解[12, 14];氯代芳香族化合物在有氧区的氧化模式主要有两种,一是先开环再脱氯,即在加氧酶的作用下,好氧微生物使苯环羟基化,形成氯代儿茶酚,进行邻位、间位开环再进行脱氯。二是先脱氯再开环,即在水解酶的作用下,氯代芳香族化合物先脱氯后再打开苯环,最终矿化[26, 72]。

|
| 图 3. 氯代烃在覆盖土中的生物降解机制模型 Figure 3. The biodegradation model of chlorinated hydrocarbon in landfill covering soil. |
Scheutz等[73]研究了填埋场覆盖层中氟氯烃 (CFCs) 的降解,发现厌氧和好氧条件下CFC-11都能发生降解,分别通过脱氯和氧化途径进行,构建的覆盖层中CFC-11降解模型与本文结论相符。
氯代烃类有机物属难降解有机物,生物处理难度大、工艺复杂,对其在复杂环境中生物降解机制的深入探究有利于开发和完善新的污染物生物降解工艺。现有研究已逐渐由单一菌种单一底物的生物降解研究向混合菌群多底物转变[74-76],同时也有研究根据不同氯代烃在好氧和厌氧条件下的降解难易程度开发了好氧/厌氧/好氧的连续降解反应器,发现对PCE、TCE、VC、三氯甲烷 (TCM)、和1, 1, 2-三氯乙烷 (1, 1, 2-TCA) 等混合氯代烃有很好的降解效果[77]。
6 复杂环境体系中氯代烃类污染生物降解研究展望量化微生物功能及相互作用关系对明晰氯代烃类污染的降解机理十分重要,然而自然环境中可培养微生物不足总量的1%,这极大限制了对复杂环境体系中氯代烃类污染物降解去除的深入研究。未来研究中,在技术上首先应广泛地采用高通量测序和宏基因组分析等先进的基因工程手段确定填埋场功能微生物在覆盖层不同深度的丰度和分布,来充分认识覆盖土中相关微生物的物种多样性、基因多样性和功能多样性。其次,利用放射性同位素标记和气质色谱等技术手段,明晰氯代烃的降解产物,深入探究氯代烃降解机理及不同深度覆盖层中多机制转化过程。这些研究不仅能够促进填埋气中氯代烃污染物的去除,也能指导其他环境介质中相关污染物的去除。
除垃圾填埋场外,氯代烃类污染物还广泛存在于多种环境介质中,包括地下水、城市污水、湖水沉积物及一些工厂周边土壤等[21, 35]。原位生物修复经济且对环境友好,是污染物的有效去除方式之一,但复杂环境中污染物降解机制认识的局限性限制了原位修复的高效应用。未来研究中可基于覆盖层中氯代烃的生物转化机制理论,首先监测出污染场地中污染物的结构、组成和含量,以确定不同污染物的最有效降解方式;第二,全面认识污染场地的微生物多样性,分析得出能够降解污染物的主导菌群;另外还要明晰污染场地不同区域的理化性质,预测相关污染物降解的程度和趋势。综合这些信息,采取合理手段来强化污染场地的原位生物修复,如污水、土壤原位修复过程中是否需要额外添加微生物、无机盐或其他的生长基质;污染水体的修复、处理过程中曝气与否,曝气时间和曝气周期的确定等相关过程。
| [1] | 孔娇艳. 三氯乙烯胁迫下垃圾生物覆盖土的甲烷氧化活性及其微生物种群结构研究. 浙江大学硕士学位论文, 2014. |
| [2] | Scheutz C, Kjeldsen P, Bogner JE, De Visscher A, Gebert J, Hilger HA, Huber-Humer M, Spokas K. Microbial methane oxidation processes and technologies for mitigation of landfill gas emissions. Waste Management & Research, 2009, 27(5): 409–455 . |
| [3] | Tchobanoglous G, Theisen H, Vigil S. Integrated solid waste management: engineering principles and management issues. Water Science and Technology Library, 1993, 8(1): 63–90 . |
| [4] | Hagedorn B, Kerfoot HB, Verwiel M, Matlock B. Geochemical and VOC-constraints on landfill gas age and attenuation characteristics: a case study from a waste disposal facility in Southern California. Waste Management, 2016, 53: 144–155 DOI:10.1016/j.wasman.2015.10.033 . |
| [5] | Xu J, Wu SJ, Xia F, Wu YM, Wang XW, Li Y, Song Z. Study on landfill gas. Environmental Science & Technology, 2007, 30(4): 48–49 (in Chinese). 徐捷, 吴诗剑, 夏凡, 吴迓名, 王歆文, 李炎, 宋钊. 垃圾填埋场挥发性有机物研究. 环境科学与技术, 2007, 30(4): 48–49. |
| [6] | Kjeldsen P, Dalager A, Broholm K. Attenuation of methane and nonmethane organic compounds in landfill gas affected soils. Journal of the Air & Waste Management Association, 1997, 47(12): 1268–1275 . |
| [7] | Allen MR, Braithwaite A, Hills CC. Trace organic compounds in landfill gas at seven U.K. waste disposal sites. Environmentl Science & Technology, 1997, 31(4): 1054–1061 . |
| [8] | Scheutz C, Mosbæk H, Kjeldsen P. Attenuation of methane and volatile organic compounds in landfill soil covers. Journal of Environmental Quality, 2004, 33(1): 61–71 DOI:10.2134/jeq2004.6100 . |
| [9] | Tassi F, Montegrossi G, Vaselli O, Liccioli C, Moretti S, Nisi B. Degradation of C2-C15 volatile organic compounds in a landfill cover soil. Science of the Total Environment, 2009, 407(15): 4513–4525 DOI:10.1016/j.scitotenv.2009.04.022 . |
| [10] | Sullivan JP, Dickinson D, Chase HA. Methanotrophs, Methylosinus trichosporium OB3b, sMMO, and their application to bioremediation. Critical Reviews in Microbiology, 1998, 24(4): 335–373 DOI:10.1080/10408419891294217 . |
| [11] | Xing ZL, Zhang LJ, Zhao TT. Advances in degradation of chlorinated hydrocarbons by obligate and facultative methanotrophs. Chinese Journal of Biotechnology, 2014, 30(4): 531–544 (in Chinese). 邢志林, 张丽杰, 赵天涛. 专一营养与兼性甲烷氧化菌降解氯代烃的研究现状、动力学分析及展望. 生物工程学报, 2014, 30(4): 531–544. |
| [12] | Schmidt M, Lege S, Nijenhuis I. Comparison of 1, 2-dichloroethane, dichloroethene and vinyl chloride carbon stable isotope fractionation during dechlorination by two Dehalococcoides strains. Water Research, 2014, 52: 146–154 DOI:10.1016/j.watres.2013.12.042 . |
| [13] | Alvarez-Cohen L, McCarty PL. Product toxicity and cometabolic competitive inhibition modeling of chloroform and trichloroethylene transformation by methanotrophic resting cells. Applied and Environmental Microbiology, 1991, 57(4): 1031–1037 . |
| [14] | Arora PK, Bae H. Bacterial degradation of chlorophenols and their derivatives. Microbial Cell Factories, 2014, 13(1): 31 DOI:10.1186/1475-2859-13-31 . |
| [15] | Cappelletti M, Frascari D, Zannoni D, Fedi S. Microbial degradation of chloroform. Applied Microbiology and Biotechnology, 2012, 96(6): 1395–1409 DOI:10.1007/s00253-012-4494-1 . |
| [16] | Hazen TC, Chakraborty R, Fleming JM, Gregory IR, Bowman JP, Jimenez L, Zhang D, Pfiffner SM, Brockman FJ, Sayler GS. Use of gene probes to assess the impact and effectiveness of aerobic in-situ bioremediation of TCE. Archives of Microbiology, 2009, 191(3): 221–232 DOI:10.1007/s00203-008-0445-8 . |
| [17] | Schuetz C, Bogner J, Chanton J, Blake D, Morcet M, Kjeldsen P. Comparative oxidation and net emissions of methane and selected non-methane organic compounds in landfill cover soils. Environmental Science & Technology, 2003, 37(22): 5150–5158 . |
| [18] | Gao YH, Zhao TT, Xing ZL, He Z, Zhang LJ, Peng XY. Effects of copper on biodegradation mechanism of trichloroethylene by mixed microorganisms. Chinese Journal of Biotechnology, 2016, 32(5): 621–634 (in Chinese). 高艳辉, 赵天涛, 邢志林, 何芝, 张丽杰, 彭绪亚. 铜离子对混合菌群降解三氯乙烯的影响与机制分析. 生物工程学报, 2016, 32(5): 621–634. |
| [19] | Im J, Moon S, Nam K, Kim YJ, Kim JY. Estimation of mass transport parameters of gases for quantifying CH4 oxidation in landfill soil covers. Waste Management, 2009, 29(2): 869–875 DOI:10.1016/j.wasman.2008.07.006 . |
| [20] | Jiang H, Chen Y, Jiang PX, Zhang C, Smith TJ, Murrell JC, Xing XH. Methanotrophs: multifunctional bacteria with promising applications in environmental bioengineering. Biochemical Engineering Journal, 2010, 49(3): 277–288 DOI:10.1016/j.bej.2010.01.003 . |
| [21] | Field J A, Sierra-Alvarez R. Biodegradability of chlorinated solvents and related chlorinated aliphatic compounds. Reviews in Environmental Science and Bio/Technology, 2004, 3(3): 185–254 DOI:10.1007/s11157-004-4733-8 . |
| [22] | Bogner JE, Chanton JP, Blake D, Abichou T, Powelson D. Effectiveness of a Florida landfill biocover for reduction of CH4 and NMHC emissions. Environmental Science & Technology, 2010, 44(4): 1197–1203 . |
| [23] | Zou SC, Lee SC, Chan CY, Ho KF, Wang XM, Chan LY, Zhang ZX. Characterization of ambient volatile organic compounds at a landfill site in Guangzhou, South China. Chemosphere, 2003, 51(9): 1015–1022 DOI:10.1016/S0045-6535(03)00004-3 . |
| [24] | Song Z. The composition and yearly variation of volatile organic compounds in ambient air around landfill area. Environmental Monitoring in China, 2013, 29(2): 98–103 (in Chinese). 宋钊. 生活垃圾填埋场空气中VOCs组成及年际变化. 中国环境监测, 2013, 29(2): 98–103. |
| [25] | Scheutz C, Bogner J, Chanton JP, Blake D, Morcet M, Aran C, Kjeldsen P. Atmospheric emissions and attenuation of non-methane organic compounds in cover soils at a French landfill. Waste Management, 2008, 28(10): 1892–1908 DOI:10.1016/j.wasman.2007.09.010 . |
| [26] | Scheutz C, Kjeldsen P. Biodegradation of trace gases in simulated landfill soil. Journal of the Air & Waste Management Association, 2005, 55(7): 878–885 . |
| [27] | Liu Y, Jiang ZA, Wang C. progress on biodegradation technology of chlorinated organics. Environmental Science & Technology, 2008, 31(2): 51–55 (in Chinese). 刘云, 蒋仲安, 王灿. 氯代有机物生物降解研究进展. 环境科学与技术, 2008, 31(2): 51–55. |
| [28] | Hazen TC. Cometabolic bioremediation//Timmis K N. Handbook of Hydrocarbon and Lipid Microbiology. Berlin Heidelberg: Springer, 2009: 2505-2514. |
| [29] | Scheutz C, Kjeldsen P. Methane oxidation and degradation of halogenated organic compounds in landfill gas affected soil [C]//Proceedings of the 1st Intercontinental Landfill Research Symposium. Luleå, Sweden, 2000. |
| [30] | Chanton J, Bogner J, Schuetz C, Blake D, Morcet M, Aran C, Kjeldsen P. Aerobic biodegradation of methane and non-methane organic compounds in landfill cover soils. 2014. |
| [31] | Muna A. Methane and non-methane organic compounds oxidation in landfill bio-covers. Doctor Dissertation of University of Ottawa (Canada), 2009. |
| [32] | Frascari D, Zanaroli G, Danko AS. In-situ aerobic cometabolism of chlorinated solvents: a review. Journal of Hazardous Materials, 2015, 283: 382–399 DOI:10.1016/j.jhazmat.2014.09.041 . |
| [33] | Wackett LP, Brusseau GA, Householder SR, Hanson RS. Survey of microbial oxygenases: trichloroethylene degradation by propane-oxidizing bacteria. Applied and Environmental Microbiology, 1989, 55(11): 2960–2964 . |
| [34] | Kim S, Hwang J, Chung J, Bae W. Enhancing trichloroethylene degradation using non-aromatic compounds as growth substrates. Journal of Hazardous Materials, 2014, 275: 99–106 DOI:10.1016/j.jhazmat.2014.04.052 . |
| [35] | Harker AR, Kim Y. Trichloroethylene degradation by two independent aromatic-degrading pathways in Alcaligenes eutrophus JMP134. Applied and Environmental Microbiology, 1990, 56(4): 1179–1181 . |
| [36] | Rasche ME, Hyman MR, Arp DJ. Factors limiting aliphatic chlorocarbon degradation by Nitrosomonas europaea: cometabolic inactivation of ammonia monooxygenase and substrate specificity. Applied and Environmental Microbiology, 1991, 57(10): 2986–2994 . |
| [37] | Hamamura N, Page C, Long T, Semprini L, Arp DJ. Chloroform cometabolism by butane-grown CF8, Pseudomonas butanovora, and Mycobacterium vaccae JOB5 and methane-grown Methylosinus trichosporium OB3b. Applied and Environmental Microbiology, 1997, 63(9): 3607–3613 . |
| [38] | Halsey KH, Sayavedra-Soto LA, Bottomley PJ, Arp DJ. Trichloroethylene degradation by butane-oxidizing bacteria causes a spectrum of toxic effects. Applied Microbiology and Biotechnology, 2005, 68(6): 794–801 DOI:10.1007/s00253-005-1944-z . |
| [39] | Tao Y, Fishman A, Bentley WE, Wood TK. Oxidation of benzene to phenol, catechol, and 1, 2, 3-trihydroxybenzene by toluene 4-monooxygenase of Pseudomonas mendocina KR1 and toluene 3-monooxygenase of Ralstonia pickettii PKO1. Applied and Environmental Microbiology, 2004, 70(7): 3814–3820 DOI:10.1128/AEM.70.7.3814-3820.2004 . |
| [40] | Olsen RH, Kukor JJ, Kaphammer B. A novel toluene-3-monooxygenase pathway cloned from Pseudomonas pickettii PKO1. Journal of Bacteriology, 1994, 176(12): 3749–3756 DOI:10.1128/jb.176.12.3749-3756.1994 . |
| [41] | Leahy JG, Byrne AM, Olsen RH. Comparison of factors influencing trichloroethylene degradation by toluene-oxidizing bacteria. Applied and Environmental Microbiology, 1996, 62(3): 825–833 . |
| [42] | Sun AK, Wood TK. Trichloroethylene degradation and mineralization by pseudomonads and Methylosinus trichosporium OB3b. Applied Microbiology and Biotechnology, 1996, 45(1/2): 248–256 . |
| [43] | Canada KA, Iwashita S, Shim H, Wood TK. Directed evolution of toluene ortho-monooxygenase for enhanced 1-naphthol synthesis and chlorinated ethene degradation. Journal of Bacteriology, 2002, 184(2): 344–349 DOI:10.1128/JB.184.2.344-349.2002 . |
| [44] | Ensign SA, Hyman MR, Arp DJ. Cometabolic degradation of chlorinated alkenes by alkene monooxygenase in a propylene-grown Xanthobacter strain. Applied and Environmental Microbiology, 1992, 58(9): 3038–3046 . |
| [45] | Ensign SA. Aliphatic and chlorinated alkenes and epoxides as inducers of alkene monooxygenase and epoxidase activities in Xanthobacter strain Py2. Applied and Environmental Microbiology, 1996, 62(1): 61–66 . |
| [46] | Saeki H, Akira M, Furuhashi K, Averhoff B, Gottschalk G. Degradation of trichloroethene by a linear-plasmid-encoded alkene monooxygenase in Rhodococcus corallinus (Nocardia corallina) B-276. Microbiology, 1999, 145(7): 1721–1730 DOI:10.1099/13500872-145-7-1721 . |
| [47] | Hartmans S, De Bont JA. Aerobic vinyl chloride metabolism in Mycobacterium aurum L1. Applied and Environmental Microbiology, 1992, 58(4): 1220–1226 . |
| [48] | Lee EH, Park H, Cho KS. Effect of substrate interaction on oxidation of methane and benzene in enriched microbial consortia from landfill cover soil. Journal of Environmental Science and Health Part A: Toxic/hazardous Substances and Environmental Engineering, 2011, 46(9): 997–1007 DOI:10.1080/10934529.2011.586266 . |
| [49] | Yao S, Xia FF, Tian BH, Li W, He R. Microbial community and function of enrichment cultures with methane and toluene. Applied Microbiology and Biotechnology, 2014, 98(7): 3121–3131 DOI:10.1007/s00253-013-5297-8 . |
| [50] | Yao S, Pei J S, Tian BH, Fan FX, Tang ML, Li W, He R. Potential application of biocover soils to landfills for mitigating toluene emission. Journal of Hazardous Materials, 2015, 299: 18–26 DOI:10.1016/j.jhazmat.2015.06.007 . |
| [51] | Kitayama A. A study on biodegradation of aromatic hydrocarbons. Doctor Dissertation of The University of Tokyo, 1997. |
| [52] | Schmidt KR, Gaza S, Voropaev A, Ertl S, Tiehm A. Aerobic biodegradation of trichloroethene without auxiliary substrates. Water Research, 2014, 59: 112–118 DOI:10.1016/j.watres.2014.04.008 . |
| [53] | Tiehm A, Schmidt KR. Sequential anaerobic/aerobic biodegradation of chloroethenes—aspects of field application. Current Opinion in Biotechnology, 2011, 22(3): 415–421 DOI:10.1016/j.copbio.2011.02.003 . |
| [54] | He Z, Zhao TT, Xing ZL, Yuan JH. Analysis of bacterial community composition in landfill cover soil. China Environmental Science, 2015, 35(12): 3744–3753 (in Chinese). 何芝, 赵天涛, 邢志林, 袁建华. 典型生活垃圾填埋场覆盖土微生物群落分析. 中国环境科学, 2015, 35(12): 3744–3753. |
| [55] | Susarla S, Masunaga S, Yonezawa Y. Reductive dechlorination pathways of chloro organics under anaerobic conditions. Water Science and Technology, 1996, 34(5/6): 489–494 . |
| [56] | Semrau JD, DiSpirito AA, Yoon S. Methanotrophs and copper. FEMS Microbiology Reviews, 2010, 34(4): 496–531 DOI:10.1111/j.1574-6976.2010.00212.x . |
| [57] | Im J, Lee SW, Bodrossy L, Barcelona MJ, Semrau JD. Field application of nitrogen and phenylacetylene to mitigate greenhouse gas emissions from landfill cover soils: effects on microbial community structure. Applied Microbiology and Biotechnology, 2011, 89(1): 189–200 DOI:10.1007/s00253-010-2811-0 . |
| [58] | Chen Y, Dumont MG, Cébron A, Murrell JC. Identification of active methanotrophs in a landfill cover soil through detection of expression of 16S rRNA and functional genes. Environmental Microbiology, 2007, 9(11): 2855–2869 DOI:10.1111/emi.2007.9.issue-11 . |
| [59] | Kumaresan D, Abell GCJ, Bodrossy L, Stralis-Pavese N, Murrell JC. Spatial and temporal diversity of methanotrophs in a landfill cover soil are differentially related to soil abiotic factors. Environmental Microbiology Reports, 2009, 1(5): 398–407 DOI:10.1111/j.1758-2229.2009.00059.x . |
| [60] | Héry M, Singer AC, Kumaresan D, Bodrossy L, Stralis-Pavese N, Prosser JI, Thompson IP, Murrell JC. Effect of earthworms on the community structure of active methanotrophic bacteria in a landfill cover soil. The ISME Journal, 2008, 2(1): 92–104 DOI:10.1038/ismej.2007.66 . |
| [61] | Cébron A, Bodrossy L, Chen Y, Singer AC, Thompson IP, Prosser JI, Murrell JC. Identity of active methanotrophs in landfill cover soil as revealed by DNA-stable isotope probing. FEMS Microbiology Ecology, 2007, 62(1): 12–23 DOI:10.1111/fem.2007.62.issue-1 . |
| [62] | Uz I, Rasche ME, Townsend T, Ogram AV, Lindner AS. Characterization of methanogenic and methanotrophic assemblages in landfill samples. Proceedings of the Royal Society B Biological Sciences, 2003, 270(S2): S202–S205 . |
| [63] | Lin B, Monreal CM, Tambong JT, Miguez CB, Carrasco-Medina L. Phylogenetic analysis of methanotrophic communities in cover soils of a landfill in Ontario. Canadian Journal of Microbiology, 2009, 55(9): 1103–1112 DOI:10.1139/W09-069 . |
| [64] | Gebert J, Singh BK, Pan Y, Bodrossy L. Activity and structure of methanotrophic communities in landfill cover soils. Environmental Microbiology Reports, 2009, 1(5): 414–423 DOI:10.1111/j.1758-2229.2009.00061.x . |
| [65] | Semrau JD. Current knowledge of microbial community structures in landfills and its cover soils. Applied Microbiology and Biotechnology, 2011, 89(4): 961–969 DOI:10.1007/s00253-010-3024-2 . |
| [66] | 赵天涛, 谭楷, 刘厚权, 邢志林, 杨旭. 可降解氯代烯烃的贪铜菌及其应用. 中国: CN104830725A. 2015-08-12. |
| [67] | 张丽杰, 何芝, 赵天涛, 全学军, 邢志林. 可降解氯代烃的甲基杆菌及其应用. 中国: CN104830727A. 2015-08-12. |
| [68] | 赵天涛, 袁建华, 李雷, 何芝, 杨旭. 降解氯代烃复合菌剂及其应用. 中国: CN104830726A. 2015-08-12. |
| [69] | Stock M, Hoefman S, Kerckhof FM, Boon I, De Vos P, De Baets B, Heylen K, Waegeman W. Exploration and prediction of interactions between methanotrophs and heterotrophs. Research in Microbiology, 2013, 164(10): 1045–1054 DOI:10.1016/j.resmic.2013.08.006 . |
| [70] | Deng Q, Li ZS, Liu TT, Feng YB. Odorous volatile organic compounds and their odor intensities in anding waste sanitary landfill in Beijing. Acta Scientiae Circumstantiae, 2016, 36(1): 201–209 (in Chinese). 邓强, 李振山, 刘添添, 冯亚斌. 北京市安定生活垃圾填埋场VOCs恶臭物质及其臭气强度. 环境科学学报, 2016, 36(1): 201–209. |
| [71] | Lu LP, Xiao WF, Liu JY, Wang J, Jiang XJ, Liu P, Zhang HJ. The biodegradation effects and mechanism of functional microbes on persistent chlorinated organic pollutants. Journal of Hangzhou Normal University (Natural Science Edition), 2014, 13(3): 298–303 (in Chinese). 鲁莉萍, 肖文丰, 刘嘉裕, 王佳, 蒋晓军, 刘鹏, 张杭君. 功能微生物对持久性氯代有机污染物的降解作用及机理. 杭州师范大学学报 (自然科学版), 2014, 13(3): 298–303. |
| [72] | Chen RR, Zhu x, Lin YS, Yu R, Long T. Preliminary inquiry of monitored natural attenuation remediation of chlorinated organic compounds contaminated sites. CIESC Journal, 2015, 66(7): 2361–2369 (in Chinese). 陈然然, 祝欣, 林玉锁, 余冉, 龙涛. 氯代有机物污染场地的监控自然衰减修复初探. 化工学报, 2015, 66(7): 2361–2369. |
| [73] | Scheutz C, Fredenslund AM, Nedenskov J, Kjeldsen P. Release and fate of fluorocarbons in a shredder residue landfill cell: 1. laboratory experiments. Waste Management, 2010, 30(11): 2153–2162 DOI:10.1016/j.wasman.2010.03.035 . |
| [74] | Zhen HJ, Du SY, Rodenburg LA, Mainelis G, Fennell DE. Reductive dechlorination of 1, 2, 3, 7, 8-pentachlorodibenzo-p-dioxin and Aroclor 1260, 1254 and 1242 by a mixed culture containing Dehalococcoides mccartyi strain 195. Water Research, 2014, 52: 51–62 DOI:10.1016/j.watres.2013.12.038 . |
| [75] | Ewald EM, Wagner A, Nijenhuis I, Richnow HH, Lechner U. Microbial dehalogenation of trichlorinated dibenzo-p-dioxins by a Dehalococcoides-containing mixed culture is coupled to carbon isotope fractionation. Environmental Science & Technology, 2007, 41(22): 7744–7751 . |
| [76] | Yu S, Dolan ME, Semprini L. Kinetics and inhibition of reductive dechlorination of chlorinated ethylenes by two different mixed cultures. Environmental Science & Technology, 2005, 39(1): 195–205 . |
| [77] | Frascari D, Fraraccio S, Nocentini M, Pinelli D. Aerobic/anaerobic/aerobic sequenced biodegradation of a mixture of chlorinated ethenes, ethanes and methanes in batch bioreactors. Bioresource Technology, 2013, 128: 479–486 DOI:10.1016/j.biortech.2012.10.026 . |
 2017, Vol. 57
2017, Vol. 57