中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- 张媛, 谢建平. 2017
- Yuan Zhang, Jianping Xie. 2017
- 结核分枝杆菌PhoP系统研究进展
- Mycobacterium tuberculosis PhoP system
- 微生物学报, 2017, 57(4): 461-467
-
文章历史
- 收稿日期:2016-08-11
- 修回日期:2016-09-21
- 网络出版日期:2016-09-26
结核分枝杆菌 (Mycobacterium tuberculosis,Mtb) 感染导致的结核病 (Tuberculosis) 仍然是全球性的重大传染病。结核分枝杆菌PhoP (Rv0757) 与PhoR (Rv0758) 组成PhoPR双组分调节系统,结核分枝杆菌基因组约2%基因的表达受其调控,包括相关毒力蛋白的表达和组成细胞壁的复杂脂质的生物合成[1-4]。本文总结了PhoP的理化性质、功能和疫苗研发等方面的研究,以及未来值得重视的研究方向。
1 理化性质Mtb的PhoP蛋白是OmpR/PhoB亚家族的一个应答调节蛋白,它的结构由N末端信号接受域和C末端DNA结合域组成,具有典型的两翼结构,图 1-A。

|
| 图 1. PhoP蛋白结构 Figure 1. Structure of PhoP protein. A: overall structure, B: ligand SO4, C: ligand PGR. Data from http://www.rcsb.org/pdb and http://www.ncbi.nlm.nih.gov. |
1.1 C末端结构
PhoP的C末端表现出典型翼状的螺旋-转角-螺旋折叠形式,结构元件被疏水核心包围,形成3股反平行β折叠且第3个螺旋具有识别功能;识别螺旋和翼残基周围的分子界面表现出强烈的正电潜能,与结合DNA有关;晶体堆积为六聚环,有头对尾形式的邻近分子相互作用,使其可以以串联的形式结合DNA[5]。此外,PhoP的C末端与Escherichia coli的PhoB (b0399) 的C末端DNA复合结构域结构重合,表明PhoP的Glu215与直接重复区域第9个碱基有特异性的作用,其识别DNA的特性可以通过蛋白质单个氨基酸的改变或DNA单个碱基的改变来调节,可能与PhoP结合特异序列DNA的机制有关[6]。
1.2 N末端结构PhoP的N末端存在特有的20氨基酸残基长度的片段,在扩大调控能力中有重要作用,与目标基因转录调节相关,可能具有调节区域结构的特点,可以由调节转录因子来响应宿主生理的改变[7]。PhoP可以通过N末端接受域形成二聚体,开关残基为Thr99和Tyr118,其中Tyr118参与形成侧链与二聚体作用的截面,其磷酸化可能促进或稳定接受区域二聚化作用[8]。有研究表明,蛋白质磷酸化不是结合DNA所必需的,但是可以通过蛋白质间相互作用增强与DNA的结合作用[9]。此外,PhoP构象的变化也与其磷酸化和结合DNA的功能有关[10](配体组成见图 1-B和C)。
2 调控底物ESX-1分泌系统是结核分枝杆菌中主要的致病效应机制之一。PhoPR双组份系统可以激活espA (Rv3616c) 调节ESX-1功能 (图 2),且EspR (ESX-1 transcriptional regulator,Rv3849) 被MprA (Rv0981) 和PhoP-Rv直接调节,因此EspR可能介导PhoPR和MprAB的调控效应,进行ESX-1功能调控[11]。PhoP调节子包括多个转录调节因子和负责聚酮体合酶、PE/PPE家族蛋白的基因,通过EspR等蛋白调控下游效应分子编码基因。当通过调节espACD操纵子控制Esx-1功能时,PhoP最突出的调节位点是位于Rv2395和PE_PGRS41之间的区间,在PhoP突变株中Mcr7 (一种ncRNA,non-coding RNA) 出现缺失同时伴随着ncRNA低水平表达[4]。此外,espACD启动子的激活需要PhoP和EspR同时存在[12]。PhoP可以调节复合脂类的生物合成,它的磷酸化可以激活编码聚酮β酮乙基合酶的基因pks2 (Rv3825c) 和pks3 (Rv1180) 基因簇的转录,并指导DNA结合在目标基因调节子区域上游[2]。吞噬体调节酸性环境的aprABC (acid and phagosome regulated) 位点的表达依赖于双组份调节因子PhoPR,缺失aprABC位点会抑制Mtb聚集和胞内生长,出现有关细胞壁脂质基因的表达缺陷,因此PhoPR可能感受吞噬体的酸性环境并且通过诱导aprABC表达来微调Mtb复合群胞内特殊的进程[13]。在受到热冲击或巨噬细胞感染后,Mtb中acr2 (Rv0251c) 的表达增加量超过其他基因,而PhoP可以调节acr2的转录,与另一个毒力调节子HspR (heat shock protein transcriptional repressor,Rv0353) 有相互作用,这两个调节子都可以独立影响acr2的表达[14]。PhoP负调节mce1 (Rv0169) 操纵子的表达[15]。此外,PhoP与目标基因的启动子结合区存在一个特殊的共有序列,有助于研究Mtb中受PhoP介导的全局调控和确定受其调控的基因[16]。
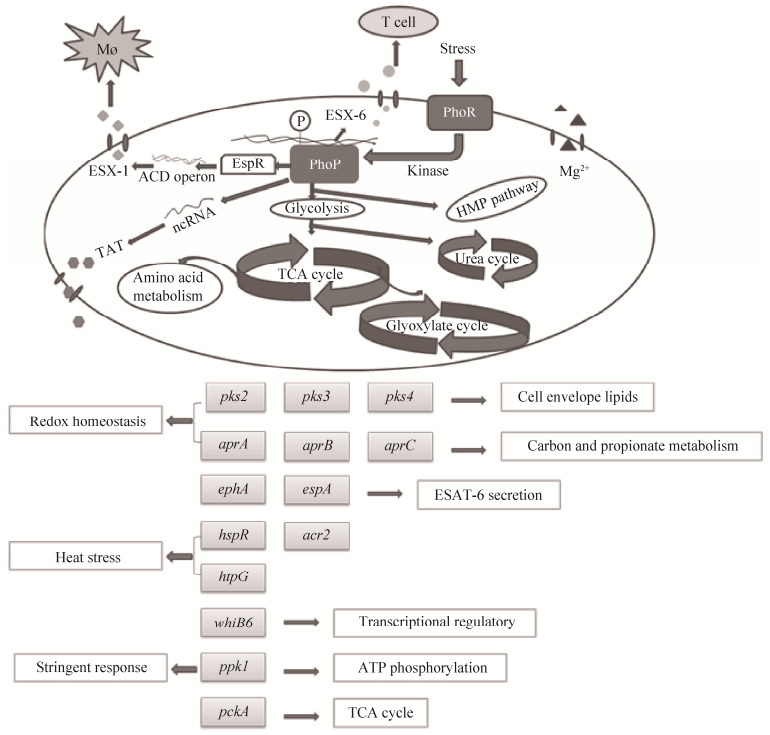
|
| 图 2. PhoP部分调控网络 Figure 2. Part of the PhoP control network. TAT, twin arginine translocation; TCA, tricarboxylic acid; MØ, macrophage. |
3 PhoP的表达调控
转录调节在结核分枝杆菌的感染中有重要作用。ArsR (Arylsulfatase R) 家族转录因子,与Mtb和Ms (Mycobacterium smegmatis) 中Rv2034与Ms6762一致,是分枝杆菌中首先发现的调节phoP的转录因子,与Ars家族中大多数调节子为负调节不同,该分子正调节Ms中phoP的表达,因此Ars转录因子可能通过调节phoPR操纵子而影响Mtb的致病能力[17]。Sigma因子SigF (Rv3286c) 也可以调控phoP[18]。无机聚磷酸盐在生物体中有着广泛的功能,结核分枝杆菌在多种压力条件下会发生聚磷酸盐的积累,在豚鼠模型中ppk1 (Rv2984) 缺失型结核菌的PhoP和SigF的转录降低[19]。此外,维生素C也可以诱导PhoP[20]。有研究发现,在鼠伤寒沙门氏菌中PhoP存在翻译后调控,Lys201可以被乙酰化和去乙酰化,且乙酰化水平的降低可以增强其结合DNA的能力[21],目前在结核分枝杆菌中没有相关报道。
4 PhoP的作用及功能通过转录组和蛋白质组学分析可知PhoP控制着许多功能,包括:通过DosR (Rv3133c) 的缺氧应答 (Rv1812c、Rv1996、Rv2628等),呼吸代谢 (Rv2329c、Rv2391、Rv2392等),应激反应 (Rv0251c、Rv0440、Rv3269等),致病性脂质的合成 (Rv1180、Rv1185c、Rv3487c等),主要T-cell抗原ESAT-6的分泌 (Rv3864、Rv3873、Rv3881c等) 和Mtb持留菌通过异柠檬酸裂解酶的转录调节等[22]。有研究称,当非致病性H37Ra和致病性H37Rv都聚集于自噬体时,后者在自噬性溶酶体中的成熟被显著抑制,且抑制有高选择性,并不扰乱巨噬细胞中基本的自噬流,这一选择性抑制功能需要毒力调节因子PhoP和ESAT-6[23]。研究发现在参与H37Ra弱毒机制的调节子PhoP的DNA结合区域中有一个点突变影响了主要T细胞抗原ESAT-6的分泌,H37Ra只转入携带来源于Mtb H37Rv的野生型phoP基因的整合粘粒表现出菌落形态、增长毒力、ESAT-6分泌和诱导特异性T细胞反应的不同,但其他H37Ra构造并没有类似现象,这一发现建立了PhoP调节子和ESAT-6分泌之间的联系,阐述了Mtb毒力调节的新观点[24]。自主吞噬在被树突细胞介导的对抗Mtb的免疫反应中有重要作用,MtbH37Ra和BCG (Bacillus Calmette-Guérin) 都不妨碍自噬体成熟,两种弱毒菌株都对其分泌的抗原靶点ESAT-6有功能性抑制作用,而这一抑制自噬体能力可以在使用来自Mtb (BCG::ESX-1) 或者PhoP (Mtb H37Ra::PhoP) 基因ESX-1区域ESAT-6分泌的调节子完全恢复[25]。此外,PhoP失活的Mtb毒力明显降低,在小鼠感染模型中会增加特异性CD4(+) T细胞,同时增加多功能细胞因子分泌[26],使小鼠存活率提高。
5 疫苗研发中的应用Mtb phoP突变株SO2与BCG相比毒力更弱,且可以增强小鼠对Mtb感染的免疫能力。Balb/c小鼠被MtbSO2与MT103感染后相比,IFN-γ(interferon-γ)、IL-4(interleukin-4) 和TNF-α (tumor necrosis factor-α) 水平较低,诱导型一氧化氮合酶 (iNOS) 在感染后期量更高,但迟发型过敏反应 (DTH) 相似[27]。在来源于单细胞的巨噬细胞和人类细胞系THP-1中,SO2增加了粘附性和胞内复制受损,阻抑吞噬体-溶酶体融合,与人巨噬细胞结合能力增强[28]。phoP突变菌有望成为预防结核病的新型活疫苗[29-30],且弱毒的SO2不会导致小鼠巨噬细胞和体内肺部感染细胞的凋亡,而有毒性的MT103会引起典型的磷脂酰丝氨酸外漏、caspase-3激活和核浓缩和碎裂的细胞凋亡[31]。MTBVAC是一种敲除phoP和fadD26 (Rv2930) 的弱毒结核分枝杆菌候选疫苗,与BCG的安全性和生物分布相同,但保护性更强,是第1个进入临床评估的结核菌减毒活疫苗[32]。erp (Rv3810) 编码分泌性含重复序列的蛋白 (ERP蛋白),参与Mtb胞内繁殖,在高度减毒的MTBVAC基础上缺失erp的菌株,在联合免疫缺陷 (SCID) 小鼠模型中的毒力比BCG和MTBVAC都低,但保护力未减,有可能用于不适合BCG的高风险人群 (如艾滋病病人)[33]。此外,phoP-phoR和mce2 (Rv0589) 操纵子敲除的牛分枝杆菌在免疫功能健全和免疫功能缺陷型小鼠中的毒力都有所降低[34]。
6 展望Mtb的PhoP-PhoR双组份系统是分枝杆菌致病和毒力的关键,可能是新的药物靶标,研究PhoP和其所结合组分,有助于鉴定不同分枝杆菌中PhoP的直接靶标[35]。利福霉素和三氯生处理可以上调phoP的基因转录3-4倍[36],利福霉素类药物为细菌DNA依赖型RNA聚合酶抑制剂,三氯生抑制脂质合成和烯酰还原酶功能,但其影响phoP转录的具体机理仍然有待研究。Mtb需要PhoPR双组份调节系统减缓其在酸性环境中生长和维持氧化还原稳态功能[37],对phop进行突变会导致Mtb生物活性脂、6 kDa抗原目标 (ESAT-6) 的分泌减少,毒力和对抗环境压力能力的降低。此外,非洲分枝杆菌L6的ESAT-6分泌不依赖PhoPR[38]。PhoP功能的多样性为今后更全面的药物和疫苗研发提供了启发。
| [1] | Ryndak M, Wang SS, Smith I. PhoP, a key player in Mycobacterium tuberculosis virulence. Trends in Microbiology, 2008, 16(11): 528–534 DOI:10.1016/j.tim.2008.08.006 . |
| [2] | Goyal R, Das AK, Singh R, Singh PK, Korpole S, Sarkar D. Phosphorylation of PhoP protein plays direct regulatory role in lipid biosynthesis of Mycobacterium tuberculosis. The Journal of Biological Chemistry, 2011, 286(52): 45197–45208 DOI:10.1074/jbc.M111.307447 . |
| [3] | Broset E, Martín C, Gonzalo-Asensio J. Evolutionary landscape of the Mycobacterium tuberculosis complex from the viewpoint of PhoPR: implications for virulence regulation and application to vaccine development. mBio, 2015, 6(5): e01289–15 . |
| [4] | Solans L, Gonzalo-Asensio J, Sala C, Benjak A, Uplekar S, Rougemont J, Guilhot C, Malaga W, Mertín C, Cole ST. The PhoP-dependent ncRNA Mcr7 modulates the TAT secretion system in Mycobacterium tuberculosis. PLoS Pathogens, 2014, 10(5): e1004183 DOI:10.1371/journal.ppat.1004183 . |
| [5] | Wang SS, Engohang-Ndong J, Smith I. Structure of the DNA-binding domain of the response regulator PhoP from Mycobacterium tuberculosis. Biochemistry, 2007, 46(51): 14751–14761 DOI:10.1021/bi700970a . |
| [6] | Das AK, Pathak A, Sinha A, Datt M, Singh B, Karthikeyan S, Sarkar D. A single-amino-acid substitution in the C terminus of PhoP determines DNA-binding specificity of the virulence-associated response regulator from Mycobacterium tuberculosis. Journal of Molecular Biology, 2010, 398(5): 647–656 DOI:10.1016/j.jmb.2010.03.056 . |
| [7] | Das AK, Kumar VA, Sevalkar RR, Bansal R, Sarkar D. Unique N-terminal arm of Mycobacterium tuberculosis PhoP protein plays an unusual role in its regulatory function. The Journal of Biological Chemistry, 2013, 288(40): 29182–29192 DOI:10.1074/jbc.M113.499905 . |
| [8] | Menon S, Wang SS. Structure of the response regulator PhoP from Mycobacterium tuberculosis reveals a dimer through the receiver domain. Biochemistry, 2011, 50(26): 5948–5957 DOI:10.1021/bi2005575 . |
| [9] | Sinha A, Gupta S, Bhutani S, Pathak A, Sarkar D. PhoP-PhoP interaction at adjacent PhoP binding sites is influenced by protein phosphorylation. Journal of Bacteriology, 2008, 190(4): 1317–1328 DOI:10.1128/JB.01074-07 . |
| [10] | Pathak A, Goyal R, Sinha A, Sarkar D. Domain structure of virulence-associated response regulator PhoP of Mycobacterium tuberculosis: role of the linker region in regulator-promoter interaction (s). The Journal of Biological Chemistry, 2010, 285(45): 34309–34318 DOI:10.1074/jbc.M110.135822 . |
| [11] | Cao GX, Howard ST, Zhang PP, Wang XH, Chen XL, Samten B, Pang XH. EspR, a regulator of the ESX-1 secretion system in Mycobacterium tuberculosis, is directly regulated by the two-component systems MprAB and PhoPR. Microbiology, 2015, 161(3): 477–489 DOI:10.1099/mic.0.000023 . |
| [12] | Kumar VA, Goyal R, Bansal R, Singh N, Sevalkar RR, Kumar A, Sarkar D. EspR-dependent ESAT-6 protein secretion of Mycobacterium tuberculosis requires the presence of virulence regulator PhoP. The Journal of Biological Chemistry, 2016, 291(36): 19018–19030 DOI:10.1074/jbc.M116.746289 . |
| [13] | Abramovitch RB, Rohde KH, Hsu FF, Russell DG. aprABC: a Mycobacterium tuberculosis complex-specific locus that modulates pH-driven adaptation to the macrophage phagosome. Molecular Microbiology, 2011, 80(3): 678–694 DOI:10.1111/mmi.2011.80.issue-3 . |
| [14] | Singh R, Kumar VA, Das AK, Bansal R, Sarkar D. A transcriptional co-repressor regulatory circuit controlling the heat-shock response of Mycobacterium tuberculosis. Molecular Microbiology, 2014, 94(2): 450–465 DOI:10.1111/mmi.2014.94.issue-2 . |
| [15] | Zeng JM, Cui T, He ZG. A genome-wide regulator-DNA interaction network in the human pathogen Mycobacterium tuberculosis H37Rv. Journal of Proteome Research, 2012, 11(9): 4682–4692 DOI:10.1021/pr3006233 . |
| [16] | Cimino M, Thomas C, Namouchi A, Dubrac S, Gicquel B, Gopaul DN. Identification of DNA binding motifs of the Mycobacterium tuberculosis PhoP/PhoR two-component signal transduction system. PLoS One, 2012, 7(8): e42876 DOI:10.1371/journal.pone.0042876 . |
| [17] | Gao CH, Yang M, He ZG. An ArsR-like transcriptional factor recognizes a conserved sequence motif and positively regulates the expression of phoP in mycobacteria. Biochemical and Biophysical Research Communication, 2011, 411(4): 726–731 DOI:10.1016/j.bbrc.2011.07.014 . |
| [18] | Hümpel A, Gebhard S, Cook GM, Berney M. The SigF regulon in Mycobacterium smegmatis reveals roles in adaptation to stationary phase, heat, and oxidative stress. Journal of Bacteriology, 2010, 192(10): 2491–2502 DOI:10.1128/JB.00035-10 . |
| [19] | Singh R, Singh M, Arora G, Kumar S, Tiwari P, Kidwai S. Polyphosphate deficiency in Mycobacterium tuberculosis is associated with enhanced drug susceptibility and impaired growth in guinea pigs. Journal of Bacteriology, 2013, 195(12): 2839–2851 DOI:10.1128/JB.00038-13 . |
| [20] | Mishra A, Sarkar D. Qualitative and quantitative proteomic analysis of vitamin C induced changes in Mycobacterium smegmatis. Frontiers in Microbiology, 2015, 6: 451 . |
| [21] | Ren J, Sang Y, Tan YC, Tao J, Ni JJ, Liu ST, Fan X, Zhao W, Lu J, Wu WJ, Yao YF. Acetylation of lysine 201 inhibits the DNA-binding ability of PhoP to regulate Salmonella virulence. PLoS Pathogens, 2016, 12(3): e1005458 DOI:10.1371/journal.ppat.1005458 . |
| [22] | Gonzalo-Asensio J, Mostowy S, Harders-Westerveen J, Huygen K, Hernández-Pando R, Thole J, Behr M, Gicquel B, Martín C. PhoP: a missing piece in the intricate puzzle of Mycobacterium tuberculosis virulence. PLoS One, 2008, 3(10): e3496 DOI:10.1371/journal.pone.0003496 . |
| [23] | Chandra P, Ghanwat S, Matta SK, Yadav SS, Mehta M, Siddiqui Z, Singh A, Kumar D. Mycobacterium tuberculosis inhibits RAB7 recruitment to selectively modulate autophagy flux in macrophages. Scientific Reports, 2015, 5: 16320 DOI:10.1038/srep16320 . |
| [24] | Frigui W, Bottai D, Majlessi L, Monot M, Josselin E, Brodin P, Garnier T, Gicquel B, Martin C, Leclerc C, Cole ST, Brosch R. Control of M. tuberculosis ESAT-6 secretion and specific T cell recognition by PhoP. PLoS Pathogens, 2008, 4(2): e33 DOI:10.1371/journal.ppat.0040033 . |
| [25] | Romagnoli A, Etna MP, Giacomini E, Pardini M, Remoli ME, Corazzari M, Falasca L, Goletti D, Gafa V, Simeone R, Delogu G, Piacentini M, Brosch R, Fimia GM, Coccia EM. ESX-1 dependent impairment of autophagic flux by Mycobacterium tuberculosis in human dendritic cells. Autophagy, 2012, 8(9): 1357–1370 DOI:10.4161/auto.20881 . |
| [26] | Nambiar JK, Pinto R, Aguilo JI, Takatsu K, Martin C, Britton WJ, Triccas JA. Protective immunity afforded by attenuated, PhoP-deficient Mycobacterium tuberculosis is associated with sustained generation of CD4+ T-cell memory. European Journal of Immunology, 2012, 42(2): 385–392 DOI:10.1002/eji.201141903 . |
| [27] | Aguilar D, Infante E, Martin C, Gormley E, Gicquel B, Hernandez Pando R. Immunological responses and protective immunity against tuberculosis conferred by vaccination of Balb/C mice with the attenuated Mycobacterium tuberculosis (phoP) SO2 strain. Clinical & Experimental Immunology, 2007, 147(2): 330–338 . |
| [28] | Ferrer NL, Gomez AB, Neyrolles O, Gicquel B, Martin C. Interactions of attenuated Mycobacterium tuberculosis phoP mutant with human macrophages. PLoS One, 2010, 5(9): e12978 DOI:10.1371/journal.pone.0012978 . |
| [29] | Asensio JAG, Arbués A, Pérez E, Gicquel B, Martin C. Live tuberculosis vaccines based on phoP mutants: a step towards clinical trials. Expert Opinion on Biological Therapy, 2008, 8(2): 201–211 DOI:10.1517/14712598.8.2.201 . |
| [30] | Cardona PJ, Asensio JG, Arbués A, Otal I, Lafoz C, Gil O, Caceres N, Ausina V, Gicquel B, Martin C. Extended safety studies of the attenuated live tuberculosis vaccine SO2 based on phoP mutant. Vaccine, 2009, 27(18): 2499–2505 DOI:10.1016/j.vaccine.2009.02.060 . |
| [31] | Aporta A, Arbues A, Aguilo JI, Monzon M, Badiola JJ, de Martino A, Ferrer N, Marinova D, Anel A, Martin C, Pardo J. Attenuated Mycobacterium tuberculosis SO2 vaccine candidate is unable to induce cell death. PLoS One, 2012, 7(9): e45213 DOI:10.1371/journal.pone.0045213 . |
| [32] | Arbues A, Aguilo JI, Gonzalo-Asensio J, Marinova D, Uranga S, Puentes E, Fernandez C, Parra A, Cardona PJ, Vilaplana C, Ausina V, Williams A, Clark S, Malaga W, Guilhot C, Gicquel B, Martin C. Construction, characterization and preclinical evaluation of MTBVAC, the first live-attenuated M. tuberculosis-based vaccine to enter clinical trials. Vaccine, 2013, 31(42): 4867–4873 DOI:10.1016/j.vaccine.2013.07.051 . |
| [33] | Solans L, Uranga S, Aguilo N, Arnal C, Gomez AB, Monzon M, Badiola JJ, Gicquel B, Martin C. Hyper-attenuated MTBVAC erp mutant protects against tuberculosis in mice. Vaccine, 2014, 32(40): 5192–5197 DOI:10.1016/j.vaccine.2014.07.047 . |
| [34] | García E, Bianco MV, Gravisaco MJ, Rocha RV, Blanco FC, Bigi F. Evaluation of Mycobacterium bovis double knockout mce2-phoP as candidate vaccine against bovine tuberculosis. Tuberculosis, 2015, 95(2): 186–189 DOI:10.1016/j.tube.2015.01.001 . |
| [35] | He XY, Wang SS. DNA consensus sequence motif for binding response regulator PhoP, a virulence regulator of Mycobacterium tuberculosis. Biochemistry, 2014, 53(51): 8008–8020 DOI:10.1021/bi501019u . |
| [36] | Boshoff HIM, Myers TG, Copp BR, McNeil MR, Wilson MA, Barry CE Ⅲ. The transcriptional responses of Mycobacterium tuberculosis to inhibitors of metabolism: novel insights into drug mechanisms of action. The Journal of Biological Chemistry, 2004, 279(38): 40174–40184 DOI:10.1074/jbc.M406796200 . |
| [37] | Baker JJ, Johnson BK, Abramovitch RB. Slow growth of Mycobacterium tuberculosis at acidic pH is regulated by phoPR and host-associated carbon sources. Molecular Microbiology, 2014, 94(1): 56–69 DOI:10.1111/mmi.2014.94.issue-1 . |
| [38] | Gonzalo-Asensio J, Malaga W, Pawlik A, Astarie-Dequeker C, Passemar C, Moreau F, Laval F, Daffé M, Martin C, Brosch R, Guilhot C. Evolutionary history of tuberculosis shaped by conserved mutations in the PhoPR virulence regulator. Proceedings of the National Acadamy of Science of the United States of America, 2014, 111(31): 11491–11496 DOI:10.1073/pnas.1406693111 . |
 2017, Vol. 57
2017, Vol. 57