中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- 蔡霞, 何进. 2017
- Xia Cai, Jin He. 2017
- 第二信使分子c-di-AMP调控细菌中钾离子转运的机制
- Second messenger c-di-AMP regulates potassium ion transport in bacteria
- 微生物学报, 57(10): 1434-1442
- Acta Microbiologica Sinica, 57(10): 1434-1442
-
文章历史
- 收稿日期:2016-11-16
- 修回日期:2017-01-06
- 网络出版日期:2017-01-19
环二腺苷酸单磷酸(cyclic diadenosine monophosphate,c-di-AMP)是新发现的第二信使分子,参与细菌多种生理功能的调节。c-di-AMP可以调节细菌对K+的转运,但目前该调控机制还没有被系统描述。本文主要综述了细菌内c-di-AMP调控K+转运的机制。
钾是生物体所必需的微量元素,主要以钾离子(K+)的形式存在于生物体中[1]。对于人体来说,长期缺钾,会有心律不整、神经传导不正常、呕吐等症状[2]。植物中钾与光合作用和呼吸作用有关,缺少钾会引起叶片收缩、发黄或出现棕褐色斑点等现象。在细菌中,K+主要维持胞内恒定的pH、渗透压和正常的膜电位[3],K+的存在还会影响胞质内酶蛋白的活力[4]。除此以外,K+还作为重要的信号分子,调节基因表达[5],参与细菌其他代谢途径的调控[6-7]。对于细菌来说,胞内K+浓度的升高被认为是细菌对外界环境中高浓度NaCl的响应[1]。
与高等动物不同,原核生物中并不存在Na+/K+ ATP泵,而细菌胞内的K+浓度通常会高于外界环境,为了控制K+的输入与输出,细菌拥有一系列的K+转运系统(K+ transport systems)、离子通道(ion channels)等来维持胞内的K+浓度。
1 细菌中的离子通道各物种拥有的K+转运系统,可能是由原始的离子通道演化而来[8-9]。依目前的研究来看,K+转运系统因能更特异、更高效地转运K+,可能会逐渐替代K+通道。将大肠杆菌(Escherichia coli)中编码K+通道的基因kch敲除后,不同的培养条件和压力都不会使突变株的生长受到影响;敲除枯草芽胞杆菌(Bacillus subtilis)的K+通道基因也不会使其产生表型变化[9];而且基因组信息显示大多数细菌中并不存在编码K+通道的基因。可能是原核生物在进化过程中,K+通道逐渐被淘汰了。
2 细菌中的K+转运系统K+转运系统在细菌转运K+的过程中起主要作用。目前,已经报道的K+转运系统有Kdp系统、Trk系统、Kup系统[10]和Ktr系统。经过深入研究,对各K+转运系统的组成及功能已较清楚。
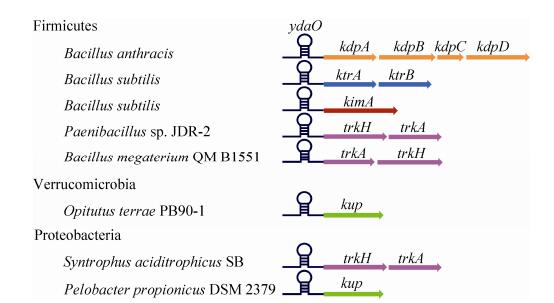
2.1 Kdp系统Kdp系统是一种可诱导、高亲和性的K+转运系统[11]。Kdp系统由双组分系统(two components system) KdpD/KdpE和KdpFABC复合物组成,KdpD是锚定在膜上的组氨酸激酶(histidine kinase),可以响应外界信号,如Na+、NH4+等[12]。当受到外界刺激时,KdpD会被磷酸化,已磷酸化的KdpD将磷酸基团传递给响应调节子(response regulator) KdpE,增加KdpE对kdp操纵子启动子区的亲和性。KdpE结合在kdp操纵子的启动子区,起始kdp操纵子的转录,最后形成KdpFABC复合物转运K+。KdpA主要负责K+的转运。K+转运过程中所需的能量由P型的ATP酶——KdpB提供,KdpB可以结合并水解ATP释放能量。KdpC通过氨基端的跨膜螺旋锚定在膜上,增加KdpB对ATP的亲和力[1, 3, 13-14]。KdpF是有单一跨膜结构域的疏水蛋白,主要维持KdpFABC复合物的稳定性[15] (图 1)。
|
| 图 1 金黄色葡萄球菌中Kdp系统的工作原理 Figure 1 The working mechanism of Kdp system in Staphylococcus aureus. The Kdp system is an inducible potassium uptake system[11], and its expression is controlled by the KdpD/KdpE two-component system. The histidine kinase KdpD is a c-di-AMP receptor, which functions together with its response regulator KdpE to activate the expression of the transporter complex KdpFABC. KdpA is a membrane component, that forms the core component of potassium channel. KdpB provides the energy required for the potassium transport by binding and hydrolyzing ATP. KdpC increases the affinity of KtrB for ATP. KdpF contains a single transmembrane domain and to enhance stability of the Kdp complex[15]. |
Kdp系统广泛分布于各类细菌中,但是不同菌株中的Kdp系统,其操纵子的组成和排列方向有差异。在酸热脂环酸杆菌(Alicyclobacillus acidocaldarius)中,KdpD被分为两部分,但是并没有失去组氨酸激酶的功能[11]。
2.2 Ktr系统Ktr系统是一种依赖Na+的K+转运系统。在菌株Synechocystis sp. strain PCC 6803中,Ktr系统是主要的K+转运系统。由KtrA、KtrB和KtrE组成。KtrB形成转运K+的核心组件,KtrA和KtrE协助KtrB特异性地运输K+[16]。在金黄色葡萄球菌(Staphylococcus aureus)中,Ktr系统由二聚体膜组分KtrB与KtrD以及胞质闸组分(gating component) KtrC或KtrA组成。Ktr系统的转运活性由结合在KtrA/KtrC上的核苷酸分子调控,当ATP结合在KtrA/KtrC上,Ktr系统转运K+的能力增强[1]。
2.3 Trk系统Trk系统是大肠杆菌转运K+的主要系统之一,相对于大肠杆菌有多种K+转运系统,海栖热袍菌(Thermotoga maritima)基因组中注释的只有1种Trk系统[17]。Trk系统是一个质子转运体,由1种跨膜蛋白和1个结合核苷酸的外周膜蛋白组成,在转运过程中需要质子动力和ATP。在结核分枝杆菌(Mycobacterium tuberculosis)中,Trk系统由2个高度同源的TrkA蛋白CeoB和CeoC组成,CeoB和CeoC由操纵子ceoBC编码。TrkA是一个亲和性较低的K+转运蛋白,在中性pH、需氧条件下转运K+[18-19]。
2.4 其他K+转运系统Kup系统与其他K+转运系统没有同源性,且Kup系统转运K+的能力很弱,在大肠杆菌中,Kup系统转运K+的能力只有Trk系统的1/10[10]。
还有一些跨膜的离子转运蛋白,例如CpaA (cation proton antiporter A)[20],也能运输K+。但对K+亲和性不高,对K+的转运没有特异性。
3 c-di-AMP调控K+的转运2008年,在解析DisA (DNA integrity scanning protein A)的晶体结构时意外发现了第二信使分子——c-di-AMP[21]。含有DAC结构域(diadenylyl cyclase domain)的二腺苷酸环化酶(diadenylate cyclase)可以利用2分子ATP合成1分子c-di-AMP[21-22]。含有DHH结构域(Asp-His-His domain)、DHH/DHHA1结构域(因与DHH结构域毗连,所以被命名为DHHA1结构域)和HD结构域(His-Asp domain)的磷酸二酯酶(phosphodiesterase)则可以将c-di-AMP降解为5′-pApA或AMP[23-24]。生物信息学分析表明DAC结构域、DHH/DHHA1结构域和HD结构域在原核生物中广泛分布,说明c-di-AMP在原核细胞中普遍存在。
c-di-AMP参与细菌多种生理功能的调节,例如调控脂肪酸代谢[25]、细菌在低盐环境的耐受性[20]、损伤DNA的修复[26]、细菌细胞壁的代谢平衡[27]、细菌的毒力[28-29]、芽胞的形成[30]以及生物被膜的产生[31]。作为第二信使分子,c-di-AMP通过与相应的受体特异性结合发挥调控作用。目前,已报道c-di-AMP的受体主要有蛋白质类以及属于RNA调控元件的核糖开关(riboswitch)两大类[32-35]。c-di-AMP通过直接与蛋白类受体结合以及与K+转运蛋白编码基因5′-非翻译区(5′-untranslated region,5′-UTR)的核糖开关结合来调控K+的转运。
3.1 c-di-AMP结合蛋白类受体调控K+转运虽然c-di-AMP被发现的较晚,但其调节功能已引起人们极大的兴趣。目前鉴定到的c-di-AMP蛋白受体越来越多,包括耻垢分枝杆菌(Mycobacterium tuberculosis)中TetR(tetracycline resistance)家族转录因子DarR[25, 33]、丙酮酸羧化酶(pyruvate carboxylase,PC)和信号转导蛋白PstA[34, 36-37],以及李斯特菌(Listeria monocytogenes)宿主的跨膜蛋白STING[38]。重要的是,c-di-AMP还与参与调控K+转运的KdpD以及K+转运蛋白KtrA、CapA和TrkA等特异性结合,从而调控K+转运[20, 39]。
c-di-AMP可以与双组分系统KdpD/KdpE中的KdpD结合。在金黄色葡萄球菌中,c-di-AMP与KdpD的USP结构域(universal stress protein domain)特异性结合[20, 39](图 1)。通过序列比对,发现KdpD广泛存在于细菌中,与金黄色葡萄球菌序列同源的KdpD分别存在于绿弯菌门(Chloroflexi)、变形菌门(Proteobacteria)、厚壁菌门(Firmicutes)、放线菌门(Actinobacteria)、浮霉状菌门(Planctomycetes)、衣原体门(Chlamydiae)、螺旋体门(Spirochaetes)、酸杆菌门(Acidobacteria)和拟杆菌门(Bacteroidetes);然而当用金黄色葡萄球菌KdpD的USP结构域作为查询序列时,KdpD的同源蛋白只存在于厚壁菌门与变形菌门中[39]。生物信息学分析结果表明,在厚壁菌门、放线菌门和部分变形菌门细菌中,存在合成c-di-AMP的DAC结构域。因此,可以推测,c-di-AMP与KdpD结合后调控kdp操纵子的表达,从而调控K+的转运,这种调控方式至少在厚壁菌门和少数变形菌门细菌中占据重要地位。例如,在金黄色葡萄球菌中高浓度的c-di-AMP抑制kdp操纵子的转录,而使细菌在低钾条件下的生存能力下降[39]。
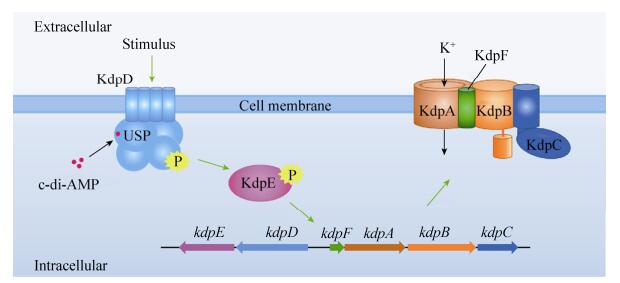
c-di-AMP可以与KtrA的RCK_C结构域结合。KtrA是Ktr系统的胞质闸组分,由氨基端的RCK_N结构域和羧基端的RCK_C结构域组成(图 2-A)[20, 40]。RCK_N结构域可以与ATP或NADH结合,为K+的转运提供能量,并与膜组分KtrB或KtrD互相作用,共同完成K+的转运[1, 20]。RCK_C结构域裸露在胞质中,可与c-di-AMP结合,调节K+的转运(图 2-A)。实验表明,金黄色葡萄球菌ktrA缺失突变株与c-di-AMP降解酶gdpP缺失突变株的生存能力均下降[20],表明高浓度的c-di-AMP不利于K+的转运。枯草芽胞杆菌、李斯特菌中c-di-AMP同样可以结合KtrA,参与K+转运的调控[18, 32]。在枯草芽胞杆菌中,5个含有RCK_C结构域的蛋白都与K+等一价离子的转运有关[32]。
|
| 图 2 c-di-AMP与K+转运系统中的蛋白结合 Figure 2 c-di-AMP binds with several K+ uptake transporters. A: c-di-AMP can bind with the RCK_C domain of KtrA; B: c-di-AMP can also bind with the RCK_C domain of CpaA; C: Similarly, c-di-AMP also binds with TrkA. |
c-di-AMP可与CpaA结合。CpaA是一个离子转运蛋白,可以将胞内的K+与Na+输送到胞外,同时向胞内输送H+。c-di-AMP也与CpaA的RCK_C结构域结合(图 2-B)[41],增加CpaA的转运活性,加速胞内K+的流失。
肺炎链球菌(Streptococcus pneumoniae)中Trk系统由TrkA与TrkH组成。最新研究发现,当TrkA与TrkH结合后,促进K+的摄取。而胞质中的c-di-AMP又可与TrkA结合(图 2-C),结合c-di-AMP的TrkA对TrkH的亲和力下降,两者的结合受到阻遏,从而影响细菌对K+的摄取[42]。高浓度的c-di-AMP抑制Trk系统对K+的转运。
c-di-AMP的蛋白类受体有很多与K+的转运相关,c-di-AMP与受体结合后,会改变受体原有的功能或活性。一般而言,高水平的c-di-AMP会抑制细菌对K+的吸收、降低胞内的K+浓度。
3.2 c-di-AMP通过结合核糖开关调控K+的转运c-di-AMP可与核糖开关特异性结合,调节下游基因的转录和翻译[35]。核糖开关是位于mRNA 5′-UTR的一种RNA元件,由适配体区(aptamer domain)和表达平台区(expression platform)组成。适配体区与特异的配体结合后,引起核糖开关构象的改变,在转录时形成终止子(terminator)或抗终止子(anti-terminator)结构或在翻译时形成隔离子(sequestor)或抗隔离子(anti-sequestor)结构,调控下游基因的转录或翻译[43-44]。
特异性响应c-di-AMP的核糖开关在Rfam中被注释为ydaO,1分子ydaO可以结合2分子c-di-AMP[45]。2004年,Breaker团队报道了ydaO,但是因没有找到合适的配体,认为其是“孤儿核糖开关”[46]。直到2013年,Breaker团队才确认ydaO的配体是c-di-AMP[47]。
ydaO广泛地分布在原核生物中,包括绿弯菌门、蓝细菌门(Cyanobacteria)、变形菌门、厚壁菌门、放线菌门、酸杆菌门、梭杆菌门(Fusobacteria)和疣微菌门(Verrucomicrobia),且下游基因功能具有多样性。这表明细菌可以通过ydaO调控多种生理功能,主要包括:细胞壁的代谢、Na+/K+的转运、芽胞的萌发及氨基酸或其他代谢物的转运等。基因组信息显示,在变形菌门、厚壁菌门和疣微菌门中,ydaO都参与调控K+的转运(图 3)[47-48]。
在厚壁菌门的不同细菌中,ydaO分别调控kdp、ktr和trk操纵子等K+转运基因的表达;变形菌门中,ydaO分别位于trk和kup操纵子的5′-UTR;疣微菌门中,ydaO调控kup基因的表达(图 3)[47]。c-di-AMP可以通过核糖开关控制K+转运蛋白基因的表达,调控K+转运。
实验研究过程中,我们发现蜡样芽胞杆菌群(Bacillus cereus group)中kdp mRNA的5′-UTR普遍存在一个ydaO。将苏云金芽胞杆菌(Bacillus thuringiensis)中的c-di-AMP合成酶敲除后的突变株ΔdisA、ΔcdaA、ΔcdaS、ΔdisAΔcdaS及ΔcdaAΔcdaS中,检测到胞内c-di-AMP浓度降低[30]。用RT-qPCR实验检测kdp操纵子转录量的变化,发现在c-di-AMP合成酶缺失株中,kdp操纵子的转录量增加。说明该ydaO为转录抑制型核糖开关,当胞内c-di-AMP的浓度低时,ydaO打开,下游基因顺利转录,形成有活性的K+转运蛋白转运K+(图 4-A)。胞内c-di-AMP浓度高时,ydaO关闭,转录终止,抑制下游基因的转录(图 4-B)。ydaO下游基因的转录受c-di-AMP浓度的严格调控。

|
| 图 4 c-di-AMP调控细菌中K+转运的机制 Figure 4 c-di-AMP regulates potassium ion transport in bacteria. A: when the intracellular c-di-AMP concentration is low, ydaO is in an open state, causing the nominal transcription of downstream gene to produce active K+ transporter; B: when the intracellular c-di-AMP level is high, ydaO binds with c-di-AMP to form a terminator, leading to transcriptional termination. |
综上所述,无论是通过蛋白受体还是核糖开关调控K+的转运,高浓度c-di-AMP都扮演抑制细菌对K+的吸收、降低胞内K+浓度的角色。
4 问题和展望K+转运的调控对细菌的生存极为重要,合适的K+浓度有助于细菌适应环境中盐浓度的改变,控制胞内酶的活性,调节胞内酸碱平衡以及保持适当的细胞膜电位。也许K+的转运还受其他胞外或胞内信号的调控,但是胞内c-di-AMP对K+转运的调节是一种非常重要的调控方式,为揭示细菌内部复杂的调控网络打开了新的一页。但是,细菌胞内含有c-di-AMP时,是否其K+转运就一定受c-di-AMP调控呢?在同一细菌中,c-di-AMP通过蛋白与核糖开关调控K+转运这两种调控方式是否同时存在?c-di-AMP是否与核糖开关结合后控制蛋白的翻译起始过程?K+浓度的变化是否影响c-di-AMP的合成?以及c-di-AMP是否有新的蛋白受体或其他新功能,都需要进一步研究。
c-di-AMP对细菌的调控功能具有一定的应用潜力,可以作为药物筛选的靶标,用于防止病菌感染;在生产应用方面,c-di-AMP调控K+转运的方式为优化菌株的培养和工作条件提供了理论基础。
| [1] | Gründling A. Potassium uptake systems in Staphylococcus aureus: new stories about ancient systems. mBio, 2013, 4(5): e00784-13. |
| [2] | Zacchia M, Abategiovanni ML, Stratigis S, Capasso G. Potassium: from physiology to clinical implications. Kidney Diseases, 2016, 2(2): 72-79. DOI:10.1159/000446268 |
| [3] | Ballal A, Basu B, Apte SK. The Kdp-ATPase system and its regulation. Journal of Biosciences, 2007, 32(3): 559-568. DOI:10.1007/s12038-007-0055-7 |
| [4] | Nanatani K, Shijuku T, Takano Y, Zulkifli L, Yamazaki T, Tominaga A, Souma S, Onai K, Morishita M, Ishiura M, Hagemann M, Suzuki I, Maruyama H, Arai F, Uozumi N. Comparative analysis of kdp and ktr mutants reveals distinct roles of the potassium transporters in the model cyanobacterium Synechocystis sp. strain PCC 6803. Journal of Bacteriology, 2015, 197(4): 676-687. DOI:10.1128/JB.02276-14 |
| [5] | Epstein W. The roles and regulation of potassium in bacteria. Progress in Nucleic Acid Research and Molecular Biology, 2003, 75: 293-320. DOI:10.1016/S0079-6603(03)75008-9 |
| [6] | Ali MK, Li X, Tang Q, Liu X, Chen F, Xiao J, Ali M, Chou SH, He J. Regulation of inducible potassium transporter KdpFABC by the KdpD/KdpE two-component system in Mycobacterium smegmatis. Frontiers in Microbiology, 2017, 8: 570. DOI:10.3389/fmicb.2017.00570 |
| [7] | Diskowski M, Mikusevic V, Stock C, H nelt I. Functional diversity of the superfamily of K+transporters to meet various requirements. Biological Chemistry, 2015, 396(9/10): 1003-1014. |
| [8] | Booth IR, Miller S, Müller A, Lehtovirta-Morley L. The evolution of bacterial mechanosensitive channels. Cell Calcium, 2015, 57(3): 140-150. DOI:10.1016/j.ceca.2014.12.011 |
| [9] | Loukin SH, Kuo MMC, Zhou XL, Haynes WJ, Kung C, Saimi Y. Microbial K+ channels. The Journal of General Physiology, 2005, 125(6): 521-527. DOI:10.1085/jgp.200509261 |
| [10] | Sato Y, Nanatani K, Hamamoto S, Shimizu M, Takahashi M, Tabuchi-Kobayashi M, Mizutani A, Schroeder JI, Souma S, Uozumi N. Defining membrane spanning domains and crucial membrane-localized acidic amino acid residues for K+transport of a Kup/HAK/KT-type Escherichia coli potassium transporter. Journal of Biochemistry, 2014, 155(5): 315-323. DOI:10.1093/jb/mvu007 |
| [11] | Huang CS, Pedersen BP, Stokes DL. Crystal structure of the potassium-importing KdpFABC membrane complex. Nature, 2017, 546(7660): 681-685. DOI:10.1038/nature22970 |
| [12] | Epstein W. The KdpD sensor kinase of Escherichia coli responds to several distinct signals to turn on expression of the Kdp transport system. Journal of Bacteriology, 2016, 198(2): 212-220. DOI:10.1128/JB.00602-15 |
| [13] | Greie JC. The KdpFABC complex from Escherichia coli: a chimeric K+transporter merging ion pumps with ion channels. European Journal of Cell Biology, 2011, 90(9): 705-710. DOI:10.1016/j.ejcb.2011.04.011 |
| [14] | Surmann K, Laermann V, Zimmann P, Altendorf K, Hammer E. Absolute quantification of the Kdp subunits of Escherichia coli by multiple reaction monitoring. Proteomics, 2014, 14(13/14): 1630-1638. |
| [15] | Gannoun-Zaki L, Belon C, Dupont C, Hilbert F, Kremer L, Blanc-Potard AB. Overexpression of the Salmonella KdpF membrane peptide modulates expression of kdp genes and intramacrophage growth. FEMS Microbiology Letters, 2014, 359(1): 34-41. DOI:10.1111/1574-6968.12559 |
| [16] | Zulkifli L, Akai M, Yoshikawa A, Shimojima M, Ohta H, Guy HR, Uozumi N. The KtrA and KtrE subunits are required for Na+-dependent K+ uptake by KtrB across the plasma membrane in Synechocystis sp. strain PCC 6803. Journal of Bacteriology, 2010, 192(19): 5063-5070. DOI:10.1128/JB.00569-10 |
| [17] | Johnson HA, Hampton E, Lesley SA. The Thermotoga maritima Trk potassium transporter——from frameshift to function. Journal of Bacteriology, 2009, 191(7): 2276-2284. DOI:10.1128/JB.01367-08 |
| [18] | Cholo MC, Boshoff HI, Steel HC, Cockeran R, Matlola NM, Downing KJ, Mizrahi V, Anderson R. Effects of clofazimine on potassium uptake by a Trk-deletion mutant of Mycobacterium tuberculosis. Journal of Antimicrobial Chemotherapy, 2006, 57(1): 79-84. |
| [19] | Cholo MC, van Rensburg EJ, Osman AG, Anderson R. Expression of the genes encoding the Trk and Kdp potassium transport systems of Mycobacterium tuberculosis during growth in vitro. Biomed Research International, 2015, 2015: 608682. |
| [20] | Corrigan RM, Campeotto I, Jeganathan T, Roelofs KG, Lee VT, Gründling A. Systematic identification of conserved bacterial c-di-AMP receptor proteins. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(22): 9084-9089. DOI:10.1073/pnas.1300595110 |
| [21] | Witte G, Hartung S, Büttner K, Hopfner KP. Structural biochemistry of a bacterial checkpoint protein reveals diadenylate cyclase activity regulated by DNA recombination intermediates. Molecular Cell, 2008, 30(2): 167-178. DOI:10.1016/j.molcel.2008.02.020 |
| [22] | Zheng C, Wang JP, Luo YC, Fu Y, Su JM, He J. Highly efficient enzymatic preparation of c-di-AMP using the diadenylate cyclase DisA from Bacillus thuringiensis. Enzyme and Microbial Technology, 2013, 52(6/7): 319-324. |
| [23] | Tang Q, Luo YC, Zheng C, Yin K, Ali MK, Li XF, He J. Functional analysis of a c-di-AMP-specific phosphodiesterase MsPDE from Mycobacterium smegmatis. International Journal of Biological Science, 2015, 11(7): 813-824. DOI:10.7150/ijbs.11797 |
| [24] | Bai YL, Yang J, Eisele LE, Underwood AJ, Koestler BJ, Waters CM, Metzger DW, Bai GC. Two DHH subfamily 1 proteins in Streptococcus pneumoniae possess cyclic di-AMP phosphodiesterase activity and affect bacterial growth and virulence. Journal of Bacteriology, 2013, 195(22): 5123-5132. DOI:10.1128/JB.00769-13 |
| [25] | Zhang L, Li WH, He ZG. DarR, a TetR-like transcriptional factor, is a cyclic di-AMP-responsive repressor in Mycobacterium smegmatis. Journal of Biological Chemistry, 2013, 288(5): 3085-3096. DOI:10.1074/jbc.M112.428110 |
| [26] | Oppenheimer-Shaanan Y, Wexselblatt E, Katzhendler J, Yavin E, Ben-Yehuda S. c-di-AMP reports DNA integrity during sporulation in Bacillus subtilis. EMBO Reports, 2011, 12(6): 594-601. DOI:10.1038/embor.2011.77 |
| [27] | Corrigan RM, Abbott JC, Burhenne H, Kaever V, Gründling A. c-di-AMP is a new second messenger in Staphylococcus aureus with a role in controlling cell size and envelope stress. PLoS Pathogens, 2011, 7(9): e1002217. DOI:10.1371/journal.ppat.1002217 |
| [28] | Cho KH, Kang SO. Streptococcus pyogenes c-di-AMP phosphodiesterase, GdpP, influences SpeB processing and virulence. PLoS One, 2013, 8(7): e69425. DOI:10.1371/journal.pone.0069425 |
| [29] | Huynh TN, Luo SK, Pensinger D, Sauer JD, Tong L, Woodward JJ. An HD-domain phosphodiesterase mediates cooperative hydrolysis of c-di-AMP to affect bacterial growth and virulence. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(7): E747-756. DOI:10.1073/pnas.1416485112 |
| [30] | Zheng C, Ma Y, Wang X, Xie YQ, Ali MK, He J. Functional analysis of the sporulation-specific diadenylate cyclase CdaS in Bacillus thuringiensis. Frontiers in Microbiology, 2015, 6: 908. |
| [31] | Gundlach J, Rath H, Herzberg C, M der U, Stülke J. Second messenger signaling in Bacillus subtilis: accumulation of cyclic di-AMP inhibits biofilm formation. Frontiers in Microbiology, 2016, 7: 804. |
| [32] | Corrigan RM, Gründling A. Cyclic di-AMP: another second messenger enters the fray. Nature Reviews Microbiology, 2013, 11(8): 513-524. DOI:10.1038/nrmicro3069 |
| [33] | Commichau FM, Dickmanns A, Gundlach J, Ficner R, Stülke J. A jack of all trades: the multiple roles of the unique essential second messenger cyclic di-AMP. Molecular Microbiology, 2015, 97(2): 189-204. DOI:10.1111/mmi.2015.97.issue-2 |
| [34] | Sureka K, Choi PH, Precit M, Delince M, Pensinger DA, Huynh TN, Jurado AR, Goo YA, Sadilek M, Iavarone AT, Sauer JD, Tong L, Woodward JJ. The cyclic dinucleotide c-di-AMP is an allosteric regulator of metabolic enzyme function. Cell, 2014, 158(6): 1389-1401. DOI:10.1016/j.cell.2014.07.046 |
| [35] | Ramesh A. Second messenger-sensing riboswitches in bacteria. Seminars in Cell & Developmental Biology, 2015, 47-48: 3-8. |
| [36] | Campeotto I, Zhang Y, Mladenov MG, Freemont PS, Gründling A. Complex structure and biochemical characterization of the Staphylococcus aureus cyclic diadenylate monophosphate (c-di-AMP)-binding protein PstA, the founding member of a new signal transduction protein family. Journal of Biological Chemistry, 2015, 290(5): 2888-2901. DOI:10.1074/jbc.M114.621789 |
| [37] | Müller M, Hopfner KP, Witte G. c-di-AMP recognition by Staphylococcus aureus PstA. FEBS Letters, 2015, 589(1): 45-51. DOI:10.1016/j.febslet.2014.11.022 |
| [38] | Woodward JJ, Iavarone AT, Portnoy DA. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science, 2010, 328(5986): 1703-1705. DOI:10.1126/science.1189801 |
| [39] | Moscoso JA, Schramke H, Zhang Y, Tosi T, Dehbi A, Jung K, Gründling A. Binding of cyclic di-AMP to the Staphylococcus aureus sensor kinase KdpD occurs via the universal stress protein domain and downregulates the expression of the Kdp potassium transporter. Journal of Bacteriology, 2016, 198(1): 98-110. DOI:10.1128/JB.00480-15 |
| [40] | Kim H, Youn SJ, Kim SO, Ko J, Lee JO, Choi BS. Structural studies of potassium transport protein KtrA regulator of conductance of K+ (RCK) C domain in complex with cyclic diadenosine monophosphate (c-di-AMP). Journal of Biological Chemistry, 2015, 290(26): 16393-16402. DOI:10.1074/jbc.M115.641340 |
| [41] | Chin KH, Liang JM, Yang JG, Shih MS, Tu ZL, Wang YC, Sun XH, Hu NJ, Liang ZX, Dow JM, Ryan PR, Chou SH. Structural insights into the distinct binding mode of cyclic di-AMP with SaCpaA_RCK. Biochemistry, 2015, 54(31): 4936-4951. DOI:10.1021/acs.biochem.5b00633 |
| [42] |
Du B, Sun JH. Cyclic diadenosine monophosphate——a new second messenger in bacteria-a review. Acta Microbiologica Sinica, 2015, 55(2): 126-133.
(in Chinese) 杜斌, 孙建和. 环二腺苷酸(c-di-AMP)——细菌的一种新型第二信使. 微生物学报, 2015, 55(2): 126-133. |
| [43] | Serganov A, Nudler E. A decade of riboswitches. Cell, 2013, 152(1/2): 17-24. |
| [44] | Breaker RR. Riboswitches and the RNA world. Cold Spring Harbor Perspectives in Biology, 2012, 4(2): a003566. |
| [45] | Jones CP, Ferré-D'Amaré AR. Crystal structure of a c-di-AMP riboswitch reveals an internally pseudo-dimeric RNA. The EMBO Journal, 2014, 33(22): 2692-2703. DOI:10.15252/embj.201489209 |
| [46] | Barrick JE, Corbino KA, Winkler WC, Nahvi A, Mandal M, Collins J, Lee M, Roth A, Sudarsan N, Jona I, Wickiser JK, Breaker RR. New RNA motifs suggest an expanded scope for riboswitches in bacterial genetic control. Proceedings of the National Academy of Sciences of the United States of America, 2004, 101(17): 6421-6426. DOI:10.1073/pnas.0308014101 |
| [47] | Nelson JW, Sudarsan N, Furukawa K, Weinberg Z, Wang JX, Breaker RR. Riboswitches in eubacteria sense the second messenger c-di-AMP. Nature Chemical Biology, 2013, 9(12): 834-839. DOI:10.1038/nchembio.1363 |
| [48] | Kellenberger CA, Chen C, Whiteley AT, Portnoy DA, Hammond MC. RNA-based fluorescent biosensors for live cell imaging of second messenger cyclic di-AMP. Journal of the American Chemical Society, 2015, 137(20): 6432-6435. DOI:10.1021/jacs.5b00275 |
 2017, Vol. 57
2017, Vol. 57